Quiz Summary
0 of 29 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 29 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 29
1. Question
1 point(s)Water is the most abundant compound on Earth.
CorrectIncorrect -
Question 2 of 29
2. Question
1 point(s)Heat from the Sun causes evaporation only from oceans, seas, and lakes.
CorrectIncorrect -
Question 3 of 29
3. Question
1 point(s)Tap water boils exactly at 100°C.
CorrectIncorrect -
Question 4 of 29
4. Question
1 point(s)The water cycle is driven by wind currents.
CorrectIncorrect -
Question 5 of 29
5. Question
1 point(s)Distilled water is used in labs because it is impure.
CorrectIncorrect -
Question 6 of 29
6. Question
1 point(s)Adding an unknown liquid to anhydrous copper(II) sulfate can test for the presence of water.
CorrectIncorrect -
Question 7 of 29
7. Question
1 point(s)Water may be electrolysed when acidified with dilute sulfuric acid, resulting in a 1:1 ratio of hydrogen to oxygen gas.
CorrectIncorrect -
Question 8 of 29
8. Question
1 point(s)Hydrogen bonds in water are stronger than covalent bonds.
CorrectIncorrect -
Question 9 of 29
9. Question
1 point(s)Water decreases in density when it freezes.
CorrectIncorrect -
Question 10 of 29
10. Question
1 point(s)Pure water is an acidic liquid.
CorrectIncorrect -
Question 11 of 29
11. Question
1 point(s)Water is an excellent solvent for ionic substances like sodium chloride.
CorrectIncorrect -
Question 12 of 29
12. Question
1 point(s)Water has a lower specific heat capacity compared to most other liquids.
CorrectIncorrect -
Question 13 of 29
13. Question
1 point(s)Boiling point of water is unusually high for a molecule of its relatively low molecular mass because of hydrogen bonding.
CorrectIncorrect -
Question 14 of 29
14. Question
1 point(s)The water used in industry and homes is returned to rivers or seas without any treatment.
CorrectIncorrect -
Question 15 of 29
15. Question
1 point(s)The majority of the Earth\’s surface is covered by water.
CorrectIncorrect -
Question 16 of 29
16. Question
1 point(s)The human skeleton contains more water by percentage than the kidneys.
CorrectIncorrect -
Question 17 of 29
17. Question
1 point(s)The sun is not involved in the water cycle on Earth.
CorrectIncorrect -
Question 18 of 29
18. Question
1 point(s)Clouds form through the condensation of water vapor.
CorrectIncorrect -
Question 19 of 29
19. Question
1 point(s)Rainwater directly enters the sea without going through rivers or lakes.
CorrectIncorrect -
Question 20 of 29
20. Question
1 point(s)Pure water has a fixed boiling point of 100°C at 1 atmosphere pressure.
CorrectIncorrect -
Question 21 of 29
21. Question
1 point(s)Tap water boils at exactly 100°C because it is pure.
CorrectIncorrect -
Question 22 of 29
22. Question
1 point(s)Distilled water is impure and contains many dissolved substances.
CorrectIncorrect -
Question 23 of 29
23. Question
1 point(s)Anhydrous copper(II) sulfate turns from white to blue if it comes in contact with water.
CorrectIncorrect -
Question 24 of 29
24. Question
1 point(s)When water is electrolyzed, the gas volume ratio at the cathode to anode is 1:2.
CorrectIncorrect -
Question 25 of 29
25. Question
1 point(s)Water is not a chemical reactant in industrial processes.
CorrectIncorrect -
Question 26 of 29
26. Question
1 point(s)Transpiration is a process where water evaporates from the surface of leaves.
CorrectIncorrect -
Question 27 of 29
27. Question
1 point(s)Reservoirs are natural bodies of water where rainwater is collected before it reaches the ocean.
CorrectIncorrect -
Question 28 of 29
28. Question
1 point(s)Water is a poor solvent and does not dissolve many substances.
CorrectIncorrect -
Question 29 of 29
29. Question
1 point(s)Acidified water with dilute sulfuric acid cannot be electrolyzed.
CorrectIncorrect
Quiz Summary
0 of 13 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 13 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 13
1. Question
1 point(s)Water must be purified before it is suitable for use in the home.
Which processes are used to remove solid impurities and bacteria?\[
\begin{array}{|c|c|c|}
\hline & \begin{array}{c}
\text { to remove } \\
\text { solid impurities }
\end{array} & \begin{array}{c}
\text { to remove } \\
\text { bacteria }
\end{array} \\
\hline \text { A } & \text { chlorination } & \text { chlorination } \\
\text { B } & \text { chlorination } & \text { filtration } \\
\text { C } & \text { filtration } & \text { chlorination } \\
\text { D } & \text { filtration } & \text { filtration } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 2 of 13
2. Question
1 point(s)The diagram shows stages in the purification of water. Which stage uses chlorine?
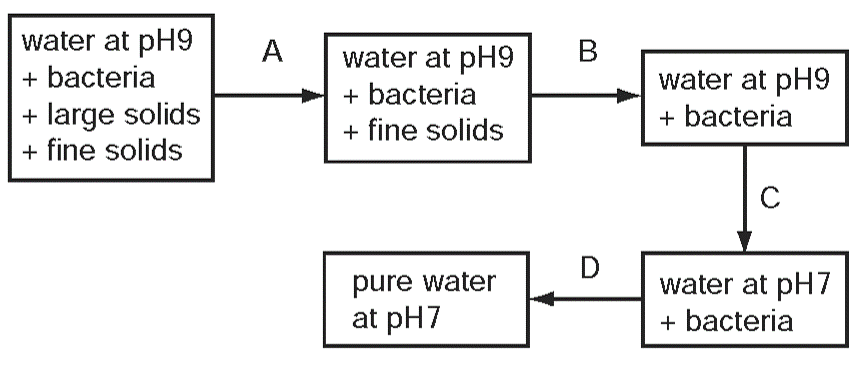 CorrectIncorrect
CorrectIncorrect -
Question 3 of 13
3. Question
1 point(s)Which statements about water are correct?
- Water is treated with chlorine to kill bacteria.
- Household water may contain salts in solution.
- Water is used in industry for cooling.
- Water for household use is filtered to remove soluble impurities.
CorrectIncorrect -
Question 4 of 13
4. Question
1 point(s)Water from a reservoir flows to the water works where purification processes 1 takes place followed by process 2. What are purification processes 1 and 2 ?
\[
\begin{array}{|c|c|c|}
\hline & \begin{array}{c}
\text { purification } \\
\text { process } 1
\end{array} & \begin{array}{c}
\text { purification } \\
\text { process } 2
\end{array} \\
\hline \text { A } & \text { chlorination } & \text { filtration } \\
\text { B } & \text { filtration } & \text { chlorination } \\
\text { C } & \text { fractional distillation } & \text { filtration } \\
\text { D } & \text { filtration } & \text { fractional distillation } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 5 of 13
5. Question
1 point(s)Some uses of water are listed.
- for drinking
- in chemical reactions
- in swimming pools
- in washing
For which uses is it necessary to chlorinate the water?
CorrectIncorrect -
Question 6 of 13
6. Question
1 point(s)Air containing an acidic impurity was neutralised by passing it through a column containing substance X.
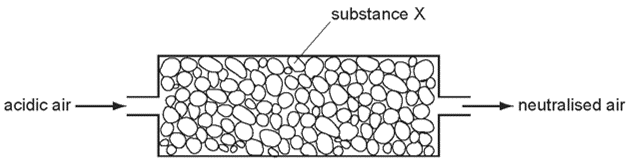
What is substance X ?CorrectIncorrect -
Question 7 of 13
7. Question
1 point(s)Water has been contaminated with sea-water.
Which substances can be removed by chlorination and filtration?CorrectIncorrect -
Question 8 of 13
8. Question
1 point(s)Water is treated at a water works to make it fit to drink.
What is present in the water when it leaves the waterworks?CorrectIncorrect -
Question 9 of 13
9. Question
1 point(s)Which method of purification would produce water most suitable for drinking?
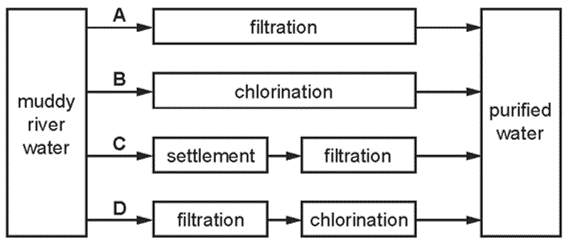 CorrectIncorrect
CorrectIncorrect -
Question 10 of 13
10. Question
1 point(s)The diagram shows stages in producing drinking water.
In which tank is chlorine added to the water?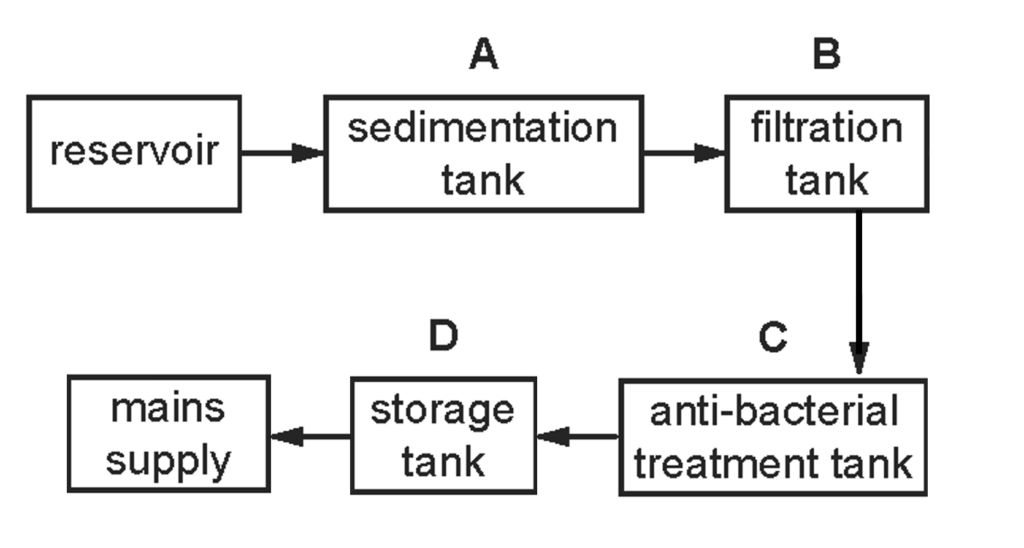 CorrectIncorrect
CorrectIncorrect -
Question 11 of 13
11. Question
1 point(s)Which statement shows that a liquid is pure water?
CorrectIncorrect -
Question 12 of 13
12. Question
1 point(s)Which substances can be used to detect the presence of water?
- cobalt(II) chloride
- copper(II) sulfate
- litmus
- methyl orange
CorrectIncorrect -
Question 13 of 13
13. Question
1 point(s)What are possible effects of an inadequate water supply during a drought?
- crop failure
- wastage of water
- human disease
- death of farm animals
CorrectIncorrect
