fuel cell structured question
2010 May-Jun Paper-43 Q5 a & c
Fuel cells are used in spacecraft to produce electrical energy.
a) How is oxygen obtained from liquid air?
Explanation: Fractional distillation separates components of liquid air at different boiling points, allowing for the extraction of oxygen.
b)Give two reasons why hydrogen may be considered to be the ideal fuel for the future:
Explanation: Burning hydrogen produces only water vapor and no greenhouse gases, and it can be produced from water, making it sustainable and environmentally friendly.
Suggest a reason why hydrogen is not widely used at the moment.
Explanation: Hydrogen gas requires high-pressure storage tanks and is not easy to handle, making its widespread use challenging and costly.
2014 Oct-Nov Paper-43 Q4
A fuel cell produces electrical energy by the oxidation of a fuel by oxygen.
The fuel is usually hydrogen but methane and methanol are two other fuels which may be used. A diagram of a hydrogen fuel cell is given below.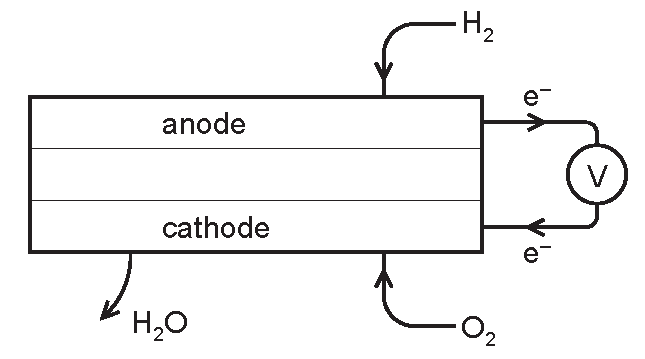
(a) When the fuel is hydrogen, the only product is water. What additional product would be formed if methane was used?
Explanation: Methane combustion typically produces both water and carbon dioxide as it contains carbon in addition to hydrogen.
(b) Write the equation for the chemical reaction that takes place in a hydrogen fuel cell.
Explanation: This equation represents the typical reaction in a hydrogen fuel cell where hydrogen gas reacts with oxygen to form water.
(c)(i)At which electrode does oxidation occur? Explain your choice.
Explanation: The anode is where oxidation occurs as hydrogen loses electrons, forming protons or hydrogen ions.
(c)(ii)Write an ionic equation for the reaction at this electrode.
Explanation: This equation shows the dissociation of hydrogen molecules into protons and electrons, demonstrating the process of oxidation at the anode.
(d)Fuel cells are used to propel cars. Give two advantages of a fuel cell over a gasoline-fuelled engine.
Explanation: Fuel cells convert chemical energy from hydrogen directly into electrical energy with higher efficiency and without harmful emissions, unlike combustion engines that emit CO₂ and other pollutants.