Quiz Summary
0 of 12 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 12 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 12
1. Question
1 point(s)A mixture of ice and water is left to stand and the ice melts.
Which row describes what happens as the ice is melting?\[
\begin{array}{|c|c|c|}
\hline & \text { temperature of mixture } & \text { energy changes } \\
\hline \text { A } & \text { increases } & \text { average kinetic energy of particles increases } \\
\text { B } & \text { increases } & \text { energy is used to overcome attractive forces } \\
\text { C } & \text { stays the same } & \text { average kinetic energy of particles increases } \\
\text { D } & \text { stays the same } & \text { energy is used to overcome attractive forces } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 2 of 12
2. Question
1 point(s)Gases are separated from liquid air by fractional distillation. The boiling points of four gases are shown.
Which gas is both monatomic and a liquid at \(-200^{\circ} \mathrm{C}\) ?\[
\begin{array}{|c|c|c|}
\hline & \text { gas } & \begin{array}{c}
\text { boiling } \\
\text { point } /{ }^{\circ} \mathrm{C}
\end{array} \\
\hline \text { A } & \text { argon } & -186 \\
\text { B } & \text { helium } & -269 \\
\text { C } & \text { neon } & -246 \\
\text { D } & \text { nitrogen } & -196 \\
\hline
\end{array}
\]CorrectIncorrect -
Question 3 of 12
3. Question
1 point(s)Which process causes the greatest increase in the distance between particles?
CorrectIncorrect -
Question 4 of 12
4. Question
1 point(s)The particles of a substance gain energy and change from a regular ordered structure to a disordered structure with large distances between the particles.
Which change of state is described?CorrectIncorrect -
Question 5 of 12
5. Question
1 point(s)In which process do particles move closer together but remain in motion?
CorrectIncorrect -
Question 6 of 12
6. Question
1 point(s)The changes that occur when a substance changes state are shown below.
\[
\text { solid } \underset{\mathrm{Z}}{\stackrel{W}{\rightleftharpoons}} \text { liquid } \underset{\mathrm{Y}}{\stackrel{X}{\rightleftharpoons}} \text { gas }
\]
Which process, \(W, X, Y\) or \(Z\), is occurring in the following four situations?
1 Butter melts on a warm day.
2 Water condenses on a cold surface.
3 The volume of liquid ethanol in an open beaker reduces.
4 Ice forms inside a freezer.\[
\begin{array}{|c|c|c|c|c|}
\hline & 1 & 2 & 3 & 4 \\
\hline \text { A } & \mathrm{W} & \mathrm{X} & \mathrm{Y} & \mathrm{Z} \\
\text { B } & \mathrm{W} & \mathrm{Y} & \mathrm{X} & \mathrm{Z} \\
\text { C } & \mathrm{X} & \mathrm{Y} & \mathrm{Z} & \mathrm{W} \\
\text { D } & \mathrm{X} & \mathrm{Z} & \mathrm{Y} & \mathrm{W} \\
\hline
\end{array}
\]CorrectIncorrect -
Question 7 of 12
7. Question
1 point(s)A liquid is heated until it boils.
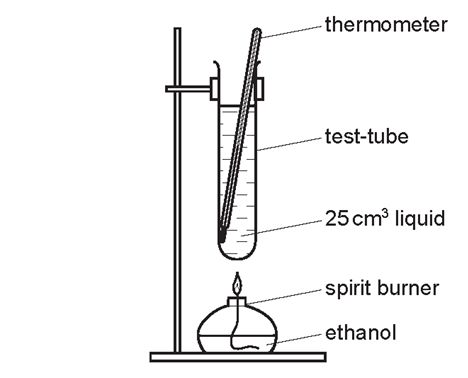
Which result shows that the liquid in the test-tube is pure water?CorrectIncorrect -
Question 8 of 12
8. Question
1 point(s)What are the processes \(\mathrm{W}, \mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\) in the following diagram?
\[
\text { solid } \underset{Y}{\rightleftharpoons} \text { liquid } \underset{Z}{\rightleftharpoons} \text { gas }
\]\[
\begin{array}{|c|c|c|c|c|}
\hline & \text { W } & \text { X } & Y & Z \\
\hline \text { A } & \text { condensing } & \text { boiling } & \text { freezing } & \text { melting } \\
\text { B } & \text { condensing } & \text { freezing } & \text { melting } & \text { boiling } \\
\text { C } & \text { melting } & \text { boiling } & \text { freezing } & \text { condensing } \\
\text { D } & \text { melting } & \text { freezing } & \text { condensing } & \text { boiling } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 9 of 12
9. Question
1 point(s)When a dark grey solid element is heated, it changes directly into a purple gas.
Which word describes this change?CorrectIncorrect -
Question 10 of 12
10. Question
1 point(s)Which statement describes sublimation?
CorrectIncorrect -
Question 11 of 12
11. Question
1 point(s)Pure water has a boiling point of \(100^{\circ} \mathrm{C}\) and a freezing point of \(0^{\circ} \mathrm{C}\).
What is the boiling point and freezing point of a sample of aqueous sodium chloride?\[
\begin{array}{|c|c|c|}
\hline & \text { boiling point } /{ }^{\circ} \mathrm{C} & \text { freezing point } /{ }^{\circ} \mathrm{C} \\
\hline \text { A } & 98 & -2 \\
\text { B } & 98 & 2 \\
\text { C } & 102 & -2 \\
\text { D } & 102 & 2 \\
\hline
\end{array}
\]CorrectIncorrect -
Question 12 of 12
12. Question
1 point(s)Impurities change the melting and boiling points of substances.
Sodium chloride is added to a sample of pure water.
How does the addition of sodium chloride affect the melting point and boiling point of the water?\[
\begin{array}{|c|c|c|}
\hline & \text { melting point } & \text { boiling point } \\
\hline \text { A } & \text { increases } & \text { increases } \\
\text { B } & \text { increases } & \text { decreases } \\
\text { C } & \text { decreases } & \text { increases } \\
\text { D } & \text { decreases } & \text { decreases } \\
\hline
\end{array}
\]CorrectIncorrect
