Radioactivity 1
0 of 15 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) A radioactive nucleus emits either an \(\alpha\)-particle or a \(\beta\)-particle.
The graph shows how the decay rate of a radioactive source changes with time. Radioactive materials should be handled carefully. A sample of a radioactive isotope has an initial rate of emission of 128 counts per minute and a half-life of 4 days. A scientist carries out an experiment using a sealed source which emits \(\beta\)-particles. The range of the \(\beta\)-particles in the air is about \(30 \mathrm{~cm}\). Which row describes the nature of \(\alpha\)-particles and of \(\gamma\)-rays? \[ The diagram shows the paths of three different types of radiation, \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\). \[ A powder contains \(400 \mathrm{mg}\) of a radioactive isotope that emits \(\alpha\)-particles. Which row gives the properties of the radiation from radioactive materials? In a laboratory, a detector of ionising radiation records an average background count rate of 8 counts per second. A radioactive source is now placed close to the detector. The count rate on the detector rises to 200 counts per second. Which statement about \(\alpha\)-radiation is correct? A radioactive source produces a count rate on a detector of 1600 counts \(/ \mathrm{s}\). \(\alpha, \beta\) and \(\gamma\)-radiations are emitted by radioactive substances. A radioactive isotope is placed near a detector. The readings on the detector are corrected for background radiation and recorded every hour. A student investigates how the radiation from a radioactive source changes with time. \[ The experiment is repeated by many other students, who also measure the count rate every two minutes.
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
What are the products of these two types of radioactive emission?
product after α-emission
product after β-emission
A
a nucleus of a different element
a nucleus of a different element
B
a nucleus of a different element
a nucleus of the same element
C
a nucleus of the same element
a nucleus of a different element
D
a nucleus of the same element
a nucleus of the same element
2. Question
1 point(s)
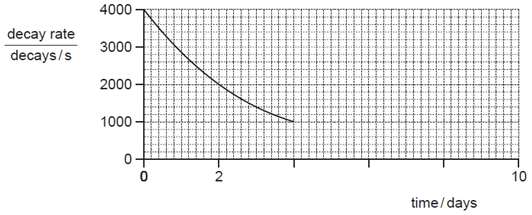
What will be the activity at 8 days?
3. Question
1 point(s)
Which safety precaution does not reduce the risk to people using a radioactive material?
4. Question
1 point(s)
How long will it take for the rate of emission to fall to 32 counts per minute?
5. Question
1 point(s)
Which precaution is the most effective to protect the scientist from the radiation?
6. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \alpha \text {-particles } & \gamma \text {-rays } \\
\hline \text { A } & \text { helium nuclei } & \text { electromagnetic radiation } \\
\text { B } & \text { helium nuclei } & \text { electrons } \\
\text { C } & \text { protons } & \text { electromagnetic radiation } \\
\text { D } & \text { protons } & \text { electrons } \\
\hline
\end{array}
\]
7. Question
1 point(s)
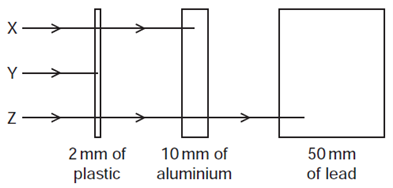
Which row in the table correctly identifies \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\) ?
\begin{array}{|c|c|c|c|}
\hline & \mathrm{X} & \mathrm{Y} & \mathrm{Z} \\
\hline \text { A } & \alpha \text {-particles } & \beta \text {-particles } & \gamma \text {-rays } \\
\text { B } & \beta \text {-particles } & \alpha \text {-particles } & \gamma \text {-rays } \\
\text { C } & \beta \text {-particles } & \gamma \text {-rays } & \alpha \text {-particles } \\
\text { D } & \gamma \text {-rays } & \alpha \text {-particles } & \beta \text {-particles } \\
\hline
\end{array}
\]
8. Question
1 point(s)
The half-life of the isotope is 5 days.
What mass of this isotope remains after 10 days?
9. Question
1 point(s)
most penetrating radiation
most highly ionising radiation
A
α
β
B
β
γ
C
γ
α
D
γ
γ
10. Question
1 point(s)
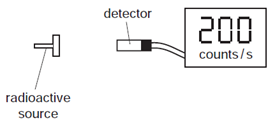
What is the count rate due to radiation from the radioactive source?
11. Question
1 point(s)
12. Question
1 point(s)
After 32 hours the count rate has fallen to 100 counts/s.
Both count rates have been corrected for background radiation.
What is the half-life of the source?
13. Question
1 point(s)
Which statement is correct?
14. Question
1 point(s)
The table shows the corrected readings.
\[
\begin{array}{|l|c|c|c|c|c|}
\hline \text { time/hours } & 0 & 1.0 & 2.0 & 3.0 & 4.0 \\
\hline \text { count rate/counts per second } & 500 & 375 & 280 & 210 & 160 \\
\hline
\end{array}
\]
What is the half-life of the isotope?
15. Question
1 point(s)
The table shows the results from the detector used by the student.
\begin{array}{c|c|}
\hline \begin{array}{c}
\text { timel } \\
\text { minutes }
\end{array} & \begin{array}{c}
\text { count ratel } \\
\text { counts per minute }
\end{array} \\
\hline 0 & 340 \\
2.0 & 180 \\
4.0 & 100 \\
6.0 & 60 \\
8.0 & 40 \\
\hline
\end{array}
\]
The half-life of the source is known to be exactly 2.0 minutes.
Why is the measured count rate always greater than half the previous value?
Radioactivity 2
0 of 17 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 17 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) A radioactive substance has a half-life of 2 weeks. At the beginning of an investigation, a sample of the substance emits \(3000 \beta\)-particles per minute. Which row shows the relative ionising effects and penetrating abilities of \(\alpha\)-particles and ß-particles? \[ The table shows the results of an experiment to find the half-life of a radioactive substance. The diagram shows a box used for storing radioactive sources. Compared with \(\beta\)-particles and \(\gamma\)-rays, \(\alpha\)-particles In a cathode-ray tube, a hot tungsten cathode releases particles by thermionic emission. The diagram shows a radioactive source, a thick aluminium sheet and a radiation detector. The count rate from a radioactive isotope is recorded every hour. The count rate is corrected for background radiation. What estimate of the half-life of the isotope can be obtained from the readings in the table? A radioactive source emits three types of radiation \(\mathrm{R}, \mathrm{S}\) and \(\mathrm{T}\). \[ The half-life of a radioactive substance is 10 minutes. A sample of the radioactive substance contains 2000 nuclei. In a cathode-ray tube, particles are fired at a screen. A tion detector is placed close to a source of \(\beta\)-particles. Eventually a sheet which is \(2.0 \mathrm{~cm}\) thick is used. The reading on the detector decreases, but does not fall to zero. A radioactive substance emits a particle from the nucleus of one of its atoms. The particle consists of two protons and two neutrons. The graph shows how the count rate on a detector due to a radioactive source changes with time. Why are some radioactive sources stored in boxes made from lead? The diagram shows the paths of three different types of radiation, \(X, Y\) and \(Z\). \[ When measuring the emissions from a radioactive rock brought into the laboratory, a teacher mentions that background radiation must be taken into account.
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
How many \(\beta\)-particles will it emit per minute after 6 weeks?
2. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { ionising effect } & \text { penetrating ability } \\
\hline \text { A } & \alpha \text { greater than } \beta & \alpha \text { greater than } \beta \\
\text { B } & \alpha \text { greater than } \beta & \alpha \text { less than } \beta \\
\text { C } & \alpha \text { less than } \beta & \alpha \text { greater than } \beta \\
\text { D } & \alpha \text { less than } \beta & \alpha \text { less than } \beta \\
\hline
\end{array}
\]
3. Question
1 point(s)
\[
\begin{array}{c|c}
\hline \text { time/s } & \frac{\text { count rate from substance }}{\text { counts / second }} \\
\hline 0 & 150 \\
60 & 120 \\
120 & 95 \\
180 & 75 \\
240 & 60 \\
\hline
\end{array}
\]
What is the half-life of the substance?
4. Question
1 point(s)
Which material is best for lining the box to prevent the escape of most radioactive emissions?
5. Question
1 point(s)
6. Question
1 point(s)
What are these particles?
7. Question
1 point(s)
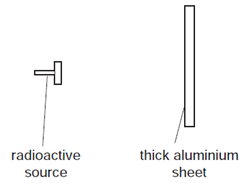

The radiation detector shows a reading greater than the background reading.
Which type of radiation is being emitted by the source and detected by the detector?
8. Question
1 point(s)
The table shows the readings.
Time/hours
Corrected count rate/counts s-1
0
800
1
620
2
480
3
370
4
290
5
220
9. Question
1 point(s)
The diagram shows an experiment set up to study the penetrating properties of \(R, S\) and \(T\).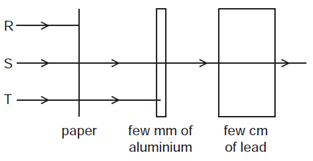
Which types of radiation are \(\mathrm{R}, \mathrm{S}\) and \(T\) ?
\begin{array}{|c|c|c|c|}
\hline & \text { R } & \mathrm{S} & \mathrm{T} \\
\hline \text { A } & \alpha \text {-particles } & \beta \text {-particles } & \gamma \text {-rays } \\
\text { B } & \alpha \text {-particles } & \gamma \text {-rays } & \beta \text {-particles } \\
\text { C } & \beta \text {-particles } & \alpha \text {-particles } & \gamma \text {-rays } \\
\text { D } & \gamma \text {-rays } & \beta \text {-particles } & \alpha \text {-particles } \\
\hline
\end{array}
\]
10. Question
1 point(s)
How many radioactive nuclei were in the sample half an hour earlier?
11. Question
1 point(s)
What are these particles?
12. Question
1 point(s)
Aluminium sheets of increasing thickness are placed between the source and the detector.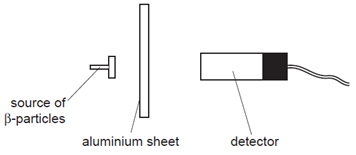
Why does the reading not fall to zero?
13. Question
1 point(s)
What is the name of this process?
14. Question
1 point(s)
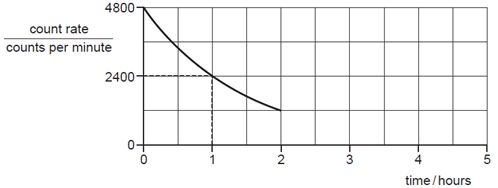
What is the count rate at 5.0 hours?
15. Question
1 point(s)
16. Question
1 point(s)
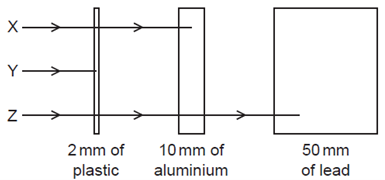
Which row in the table correctly identifies \(\mathrm{X}, \mathrm{Y}\) and \(\mathrm{Z}\) ?
\begin{array}{|c|c|c|c|}
\hline & \mathbf{X} & \mathrm{Y} & Z \\
\hline \text { A } & \alpha \text {-particles } & \beta \text {-particles } & \gamma \text {-rays } \\
\text { B } & \beta \text {-particles } & \alpha \text {-particles } & \gamma \text {-rays } \\
\text { C } & \beta \text {-particles } & \gamma \text {-rays } & \alpha \text {-particles } \\
\text { D } & \gamma \text {-rays } & \alpha \text {-particles } & \beta \text {-particles } \\
\hline
\end{array}
\]
17. Question
1 point(s)
What is this background radiation?
Radioactivity 3
0 of 15 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Which row shows the relative ionising effects and penetrating abilities of \(\alpha\)-particles and \(\beta\)-particles? \[ A powder contains \(400 \mathrm{mg}\) of a radioactive material that emits \(\alpha\)-particles. A scientist needs to use a source of \(\gamma\)-rays as safely as possible. The graph shows the activity of a radioactive source over a period of time. The arrangement shown is used to check whether the flour inside a cardboard packet is above a certain level. If it is above this level, the flour absorbs the radiation from the source so that it doesn’t reach the detector. A reading is taken every 10 minutes of the number of emissions per second from a radioactive source. The table shows the readings. A radioactive decay can be represented as shown. The graph shows how the decay rate of a radioactive source changes with time. Sodium-24 decays to magnesium-24 according to the following equation. The reading on a detector placed near a radioactive material is 536 counts per second. A nucleus of a radioactive substance \({ }_{84}^{218} \mathrm{Po}\) undergoes an \(\alpha\)-decay followed by a \(\beta\)-decay. \[ Which row describes the nature of \(\alpha\)-particles and of \(\gamma\)-rays? \[ A beam of \(\gamma\)-rays passes between two charged metal plates as shown in the diagram. How do the \(\gamma\)-rays pass between the two charged plates? A powder contains \(400 \mathrm{mg}\) of a radioactive isotope that emits \(\alpha\)-particles. A scientist carries out an experiment using a sealed source which emits \(\beta\)-particles. The range of the \(\beta\)-particles in the air is about \(30 \mathrm{~cm}\).
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { ionising effect } & \text { penetrating ability } \\
\hline \text { A } & \alpha \text { greater than } \beta & \alpha \text { greater than } \beta \\
\text { B } & \alpha \text { greater than } \beta & \alpha \text { less than } \beta \\
\text { C } & \alpha \text { less than } \beta & \alpha \text { greater than } \beta \\
\text { D } & \alpha \text { less than } \beta & \alpha \text { less than } \beta \\
\hline
\end{array}
\]
2. Question
1 point(s)
The half-life of the material is 5 days.
What mass of that material remains after 10 days?
3. Question
1 point(s)
Which action will not reduce the amount of radiation that reaches the scientist?
4. Question
1 point(s)
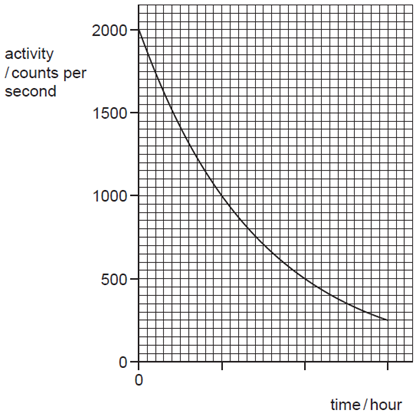
What is the half-life of the source?
5. Question
1 point(s)
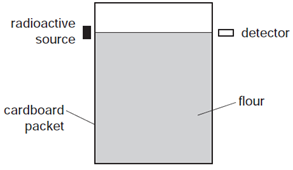
Which type of radiation is suitable to use?
6. Question
1 point(s)
\[
\begin{array}{|c|c|}
\hline \text { time/min } & \begin{array}{c}
\text { number of } \\
\text { emissions } \\
\text { per second }
\end{array} \\
\hline 0 & 800 \\
10 & 560 \\
20 & 400 \\
30 & 280 \\
40 & 200 \\
50 & 140 \\
60 & 100 \\
\hline
\end{array}
\]
What is the half-life of the source?
7. Question
1 point(s)
\[
{ }_{91}^{233} \mathrm{~Pa} \rightarrow{ }_{92}^{233} \mathrm{U}
\]
The equation is incomplete.
In this decay, the nucleus changes by
8. Question
1 point(s)
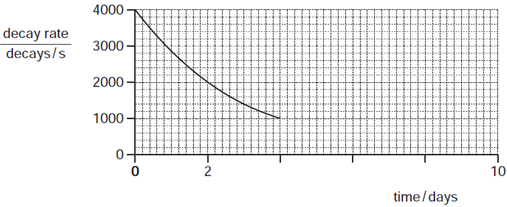
What will be the decay rate at 8 days?
9. Question
1 point(s)
\[
{ }_{11}^{24} \mathrm{Na} \rightarrow{ }_{12}^{24} \mathrm{Mg}+\text { emitted particle }
\]
What is the emitted particle?
10. Question
1 point(s)
The background count rate is 44 counts per second.
The half-life of the radioactive material is 34 hours.
What is the reading on the detector after 68 hours?
11. Question
1 point(s)
What are the nucleon (mass) number and proton (atomic) number of the nuclide formed after both decays have happened?
\begin{array}{c|c|c}
& \text { nucleon number } & \text { proton number } \\
\hline \text { A } & 214 & 85 \\
\text { B } & 216 & 85 \\
\text { C } & 214 & 83 \\
\text { D } & 216 & 83 \\
\hline
\end{array}
\]
12. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \alpha \text {-particles } & \gamma \text {-rays } \\
\hline \text { A } & \text { helium nuclei } & \text { electromagnetic radiation } \\
\text { B } & \text { helium nuclei } & \text { electrons } \\
\text { C } & \text { protons } & \text { electromagnetic radiation } \\
\text { D } & \text { protons } & \text { electrons } \\
\hline
\end{array}
\]
13. Question
1 point(s)
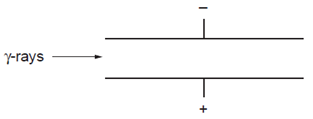
14. Question
1 point(s)
The half-life of the isotope is 5 days.
What mass of this isotope remains after 10 days?
15. Question
1 point(s)
Which precaution is the most effective to protect the scientist from the radiation?
