Quiz Summary
0 of 8 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 8 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 8
1. Question
1 point(s)Diamond is extremely hard and does not conduct electricity. Which statement explains these properties?
CorrectIncorrect -
Question 2 of 8
2. Question
1 point(s)Two statements about silicon (IV) oxide are given.
- It is a hard substance.
- It has a macromolecular structure with strong covalent bonds.
Which is correct?
CorrectIncorrect -
Question 3 of 8
3. Question
1 point(s)X is a solid at room temperature. X has a high melting point. Solid X conducts electricity. Which diagram shows how the particles are arranged in solid X?
CorrectIncorrect -
Question 4 of 8
4. Question
1 point(s)Rescuers are drilling through fallen rock in order to rescue some men trapped in a cave. The drill needs lubricating from time to time.
The following statements were made about the materials used for the drill tip and the lubricant and the reasons for their use. Which statements are correct?CorrectIncorrect -
Question 5 of 8
5. Question
1 point(s)The ‘lead’ in a pencil is made of a mixture of graphite and clay.
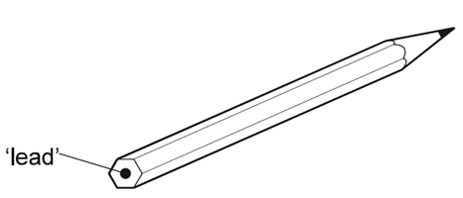
When the percentage of graphite is increased, the pencil slides across the paper more easily.
Which statement explains this observation?CorrectIncorrect -
Question 6 of 8
6. Question
1 point(s)- Solid F is an element.
- Solid G is a compound.
- Neither solid conducts electricity but G conducts electricity when dissolved in water.
These properties suggest that F is ._(1)_ and that G is _(2)_ with _(3)_ bonds.
Which words correctly complete gaps 1, 2 and 3?
CorrectIncorrect -
Question 7 of 8
7. Question
1 point(s)Slate has a layered structure and can easily be split into thin sheets. Which diagram shows a structure most like that of slate?
CorrectIncorrect -
Question 8 of 8
8. Question
1 point(s)Sodium chloride is an ionic solid. Which statement is not correct?
CorrectIncorrect
Quiz Summary
0 of 6 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 6 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 6
1. Question
1 point(s)Element \(X\) is shiny and can be formed into a sheet by hammering.
Which row correctly describes the properties of element \(X\) ?\[
\begin{array}{|c|c|c|}
\hline & \text { conducts electricity } & \text { melts below } 25^{\circ} \mathrm{C} \\
\hline \text { A } & \checkmark & \checkmark \\
\text { B } & \checkmark & ✘ \\
\text { C } & ✘ & \checkmark \\
\text { D } & ✘ & ✘ \\
\hline
\end{array}
\]CorrectIncorrect -
Question 2 of 6
2. Question
1 point(s)The diagram represents the general structure of a solid Z. What is Z?
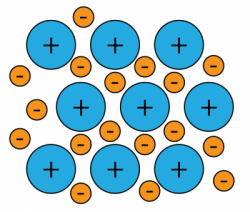 CorrectIncorrect
CorrectIncorrect -
Question 3 of 6
3. Question
1 point(s)Substance P conducts electricity when solid.
Which particles move in solid \(P\) so that it can conduct electricity?- anions
- cations
- electrons
CorrectIncorrect -
Question 4 of 6
4. Question
1 point(s)Which particles are present in the structure of metals?
- positive ions
- negative ions
- shared pairs of electrons
- mobile electrons
CorrectIncorrect -
Question 5 of 6
5. Question
1 point(s)Which type of structure and bonding is present in an element that is malleable and conducts electricity?
CorrectIncorrect -
Question 6 of 6
6. Question
1 point(s)Which statement explains why metals are malleable?
CorrectIncorrect
