2009 May-Jun Paper-41 Q7a
Hydrogen reacts with the halogens to form hydrogen halides.
(a) Bond energy is the amount of energy, in \(\mathrm{kJ}\), that must be supplied (endothermic) to break one mole of a bond.
\begin{align}
\begin{array}{|c|c|}
\hline \text { bond } & \text { bond energy in kJ/mol } \\
\hline \mathrm{H}-\mathrm{H} & +436 \\
\hline \mathrm{Cl}-\mathrm{Cl} & +242 \\
\hline \mathrm{H}-\mathrm{Cl} & +431 \\
\hline
\end{array}
\end{align}
Use the above data to show that the following reaction is exothermic:
\[ \text{H-H} + \text{Cl-Cl} \rightarrow 2\text{H-Cl} \]
total energy released = 2 × (-431) = – 862 kJ
change for reaction = +687kJ + (-862kJ) = -184kJ
The reaction is exothermic with an energy released 184 kJ more than energy absorbed.
Explanation: The total energy required to break the H-H and Cl-Cl bonds is 678 kJ (endothermic), while the energy released from forming two H-Cl bonds is 862 kJ (exothermic), resulting in a net energy release of 184 kJ.
2010 May-Jun Paper-43 Q5 b
Hydrogen and oxygen react to form water.
\[
2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}
\]
(i) Give an example of bond breaking in the above reaction.
Explanation: During the formation of water, the double bond between the oxygen atoms in the oxygen molecule must break to allow oxygen to bond with hydrogen.
(ii)Give an example of bond forming in the above reaction.**
Explanation: After the oxygen and hydrogen bonds break, new bonds form between the oxygen and hydrogen atoms to create water molecules.
(iii) Is the change given in (i) exothermic or endothermic?
Explanation: The formation of water from hydrogen and oxygen releases energy, making it an exothermic process. The bond energy of the newly formed O-H bonds in water is greater than the energy required to break the O=O and H-H bonds, thus releasing heat.
2014 May-Jun Paper-43 Q5 e
Ammonia is used to make nitrogen trifluoride, NF₃, which is essential for the electronics industry. It is made by the following reaction.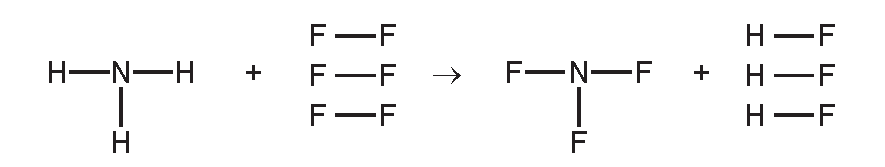
Determine if the reaction is exothermic or endothermic using the following bond energies and by completing the following table. The first line has been done as an example. Bond energy is the amount of energy, in \(\mathrm{kJ} /\) mole, needed to break or make one mole of the bond.
\begin{align}
\begin{array}{|c|c|}
\hline \text { bond } & \text { bond energy in } \mathrm{kJ} / \text { mole } \\
\hline \mathrm{N}-\mathrm{H} & 390 \\
\hline \mathrm{F}-\mathrm{F} & 155 \\
\hline \mathrm{N}-\mathrm{F} & 280 \\
\hline \mathrm{H}-\mathrm{F} & 565 \\
\hline
\end{array}
\end{align}
\begin{align}
\begin{array}{|c|c|}
\hline \text { bond } & \text { energy change } / \mathrm{kJ} \\
\hline \mathrm{N}-\mathrm{H} & (3 \times 390)=1170 \\
\hline \mathrm{F}-\mathrm{F} & \\
\hline \mathrm{N}-\mathrm{F} & \\
\hline \mathrm{H}-\mathrm{F} & \\
\hline
\end{array}
\end{align}
\begin{align}
\begin{array}{|l|l|l|}
\hline \text { Bond } & \text { Bond Energy (kJ/mole) } & \text { Energy Change (kJ) } \\
\hline \text { N-H } & 390 & (3 \times 390)=1170 \\
\hline \text { F-F } & 155 & +3 \times 155=+465 \\
\hline \text { N-F } & 280 & -3 \times 280=-840 \\
\hline \text { H-F } & 565 & -3 \times 565=-1695 \\
\hline
\end{array}
\end{align}
The reaction is exothermic.
Explanation: The total energy required to break the bonds (N—H and F—F) is 1635 kJ, while the energy released from forming the bonds (N—F and H—F) is 2535 kJ, leading to a net release of energy.
2015 May-Jun Paper-43 Q6 c-d
(c) Give an equation for the complete combustion of propane.
Explanation: Propane (\(\text{C}_3\text{H}_8\)) combusts with oxygen (\(\text{O}_2\)) to produce carbon dioxide (\(\text{CO}_2\)) and water (\(\text{H}_2\text{O}\)), releasing energy in the process, indicating it is exothermic.
(d)(i) Where does the energy for photosynthesis come from?
Explanation: Sunlight provides the energy needed for plants to convert carbon dioxide and water into glucose and oxygen.
(d)(ii) Give the word equation for photosynthesis.
Explanation: This equation summarizes the process by which plants use the energy from sunlight to convert carbon dioxide and water into glucose and oxygen, essential for their growth and oxygen release into the atmosphere.
2016 May-Jun Paper-41 Q2 f(i)
(f) Sulfur tetrafluoride, \(\mathrm{SF}_4\), can be made by combining gaseous sulfur with fluorine.
\[
\mathrm{S}(\mathrm{g})+2 \mathrm{~F}_2(\mathrm{~g}) \rightarrow \mathrm{SF}_4(\mathrm{~g})
\]
The reaction is exothermic.
(i) Complete the energy level diagram for the reaction of sulfur with fluorine to form sulfur tetrafluoride (SF₄), which is exothermic. Include an arrow showing the energy change during the reaction.
– The reactants (\(\text{S}(g) + 2\text{F}_2(g)\)) are placed on the reactant line.
– The product (\(\text{SF}_4(g)\)) is placed on a product line that is lower than the reactant line, indicating the release of energy.
– An arrow starts at the reactant energy level and ends at the product energy level, pointing downwards to show the exothermic nature of the reaction, indicating that energy is released as the reaction proceeds.
Explanation: The diagram illustrates that the formation of SF₄ from sulfur and fluorine involves a decrease in energy, characteristic of exothermic reactions, where the products have lower energy than the reactants.
2017 Oct-Nov Paper-42 Q3 a-b
The chemical equation for the complete combustion of ethanol, \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}\), is shown.
\[
\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+3 \mathrm{H}_2 \mathrm{O}
\]
The energy released when one mole of ethanol undergoes complete combustion is \(1280 \mathrm{KJ}\).
Part of the energy level diagram for this reaction is shown. (a) Complete the energy level diagram to show the products of the reaction and the overall energy change of the reaction.
(a) Complete the energy level diagram to show the products of the reaction and the overall energy change of the reaction.
– An arrow should be drawn starting from the energy level of the reactants (C₂H₅OH + 3O₂) and finishing at the energy level of the products (2CO₂ + 3H₂O). This arrow should point downwards, showing that the energy of the products is less than that of the reactants, indicating energy is released.
Explanation: This arrangement highlights the exothermic reaction where 1280 kJ is released per mole of ethanol combusted, demonstrating a decrease in energy from reactants to products.
(b) What does X represent?
Explanation: Activation energy is the minimum energy barrier that must be overcome for the reactants to transform into products. It signifies the peak energy state before the reaction proceeds to form the final products.
2019 May-Jun Paper-43 Q5 b
(b) The reaction between ethanoic acid and ethanol is exothermic.
Draw an energy level diagram for this reaction.
On your diagram label:
– the reactants and products
– the energy change of the reaction, \(\Delta H\).
– M2 (Reactants and product positions identified): The diagram should clearly label where the reactants (ethanoic acid and ethanol) and the products are located.
– M3 (Energy change shown as approximately vertical line indicating gap between reactants and products with arrow head pointing from reactant to products. Arrow needs to be labelled):This describes the energy change (ΔH) which should be shown as a downward arrow, indicating that energy is released, moving from a higher to a lower energy state.
Explanation: In an exothermic reaction, the energy required to break the bonds in the reactants is less than the energy released when new bonds form in the products, resulting in an overall release of energy as shown by the lower energy level of the products compared to the reactants.
