0 of 9 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 9 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) What is the empirical formula of an oxide of iron, formed by reacting \(2.24 \mathrm{~g}\) of iron with \(0.96 \mathrm{~g}\) of oxygen? An experiment was done to determine the formula of a hydrocarbon, \(\mathrm{C}_{\mathrm{x}} \mathrm{H}_{\mathrm{y}}\). A compound contains \(34.5 \%\) calcium, \(24.1 \%\) silicon and \(41.4 \%\) oxygen by mass. The relative molecular mass of an alcohol is 88 . \[ Benzene is a liquid with molecular formula \(\mathrm{C}_6 \mathrm{H}_6\). Analysis of a compound formed between magnesium and nitrogen showed it contained \(14.4 \mathrm{~g}\) of magnesium and \(5.6 \mathrm{~g}\) of nitrogen. In athletics, banned drugs such as nandrolone have been taken illegally to improve performance. Nandrolone has the molecular formula \(\mathrm{C}_{18} \mathrm{H}_{26} \mathrm{O}_2\). What is the relative molecular mass, \(M_r\), of nitrogen dioxide? The diagram shows an experiment to find the formula of magnesium oxide.
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
\(10 \mathrm{~cm}^3\) of the gaseous hydrocarbon, \(\mathrm{C}_x \mathrm{H}_y\), was burned in an excess of oxygen to form \(20 \mathrm{~cm}^3\) of carbon dioxide and \(30 \mathrm{~cm}^3\) of water vapour.
What is \(\mathrm{C}_{\mathrm{x}} \mathrm{H}_{\mathrm{y}}\) ?
3. Question
1 point(s)
What is its empirical formula?
4. Question
1 point(s)
Its percentage composition by mass is: \(\mathrm{C}, 54.5 \% ; \mathrm{H}, 9.1 \% ; \mathrm{O}, 36.4 \%\).
Which row shows the empirical formula and molecular formula for this alcohol?
\begin{array}{|c|c|c|}
\hline & \text { empirical formula } & \text { molecular formula } \\
\hline \text { A } & \mathrm{C}_2 \mathrm{H}_4 \mathrm{O} & \mathrm{C}_2 \mathrm{H}_4 \mathrm{O} \\
\text { B } & \mathrm{C}_2 \mathrm{H}_4 \mathrm{O} & \mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_2 \\
\text { C } & \mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_2 & \mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_2 \\
\text { D } & \mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_2 & \mathrm{C}_2 \mathrm{H}_4 \mathrm{O} \\
\hline
\end{array}
\]
5. Question
1 point(s)
Ethene is a gas with molecular formula \(\mathrm{C}_2 \mathrm{H}_4\).
Which statement is correct?
6. Question
1 point(s)
What is the empirical formula of the compound?
7. Question
1 point(s)
What is the relative molecular mass, \(M_r\), of nandrolone?
(Relative atomic mass: \(\mathrm{H}=1 ; \mathrm{C}=12 ; \mathrm{O}=16\) )
8. Question
1 point(s)
9. Question
1 point(s)
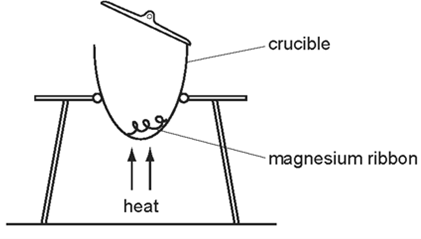
Which piece of apparatus would be needed in addition to those shown?
Chemical Formulae
