Quiz Summary
0 of 7 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 7 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 7
1. Question
1 point(s)Which of the following is correct about the product at the anode and cathode for the electrolysis of molten potassium bromide?
CorrectIncorrect -
Question 2 of 7
2. Question
1 point(s)Which of these elements could be formed at the anode when a molten salt is electrolysed?
CorrectIncorrect -
Question 3 of 7
3. Question
1 point(s)What are the electrode products when molten silver iodide is electrolysed between inert electrodes?
\[
\begin{array}{|c|c|c|}
\hline & \text { cathode } & \text { anode } \\
\hline \text { A } & \text { hydrogen } & \text { iodine } \\
\text { B } & \text { iodine } & \text { silver } \\
\text { C } & \text { silver } & \text { iodine } \\
\text { D } & \text { silver } & \text { oxygen } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 4 of 7
4. Question
1 point(s)Which statement about the electrolysis of molten lead(II) bromide is correct?
CorrectIncorrect -
Question 5 of 7
5. Question
1 point(s)What is the ionic half-equation for the reaction that occurs at the cathode when molten lead(II) bromide is electrolysed?
CorrectIncorrect -
Question 6 of 7
6. Question
1 point(s)Concentrated aqueous potassium bromide solution is electrolysed using inert electrodes.
The ions present in the solution are \(\mathrm{K}^{+}, \mathrm{Br}^{-}, \mathrm{H}^{+}\)and \(\mathrm{OH}^{-}\).
To which electrodes are the ions attracted during this electrolysis?\[
\begin{array}{|c|c|c|}
\hline & \text { attracted to anode } & \text { attracted to cathode } \\
\hline \text { A } & \mathrm{Br}^{-} \text {and } \mathrm{K}^{+} & \mathrm{H}^{+} \text {and } \mathrm{OH}^{-} \\
\text {B } & \mathrm{Br}^{-} \text {and } \mathrm{OH}^{-} & \mathrm{H}^{+} \text {and } \mathrm{K}^{+} \\
\text {C } & \mathrm{H}^{+} \text {and } \mathrm{K}^{+} & \mathrm{Br}^{-} \text {and } \mathrm{OH}^{-} \\
\text {D } & \mathrm{H}^{+} \text {and } \mathrm{OH}^{-} & \mathrm{Br}^{-} \text {and K } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 7 of 7
7. Question
1 point(s)Aqueous copper(II) sulfate solution is electrolysed using inert electrodes.
Copper(II) ions \(\left(\mathrm{Cu}^{2+}\right)\), hydrogen ions \(\left(\mathrm{H}^{+}\right)\), hydroxide ions \(\left(\mathrm{OH}^{-}\right)\)and sulfate ions \(\left(\mathrm{SO}_4^{2-}\right)\) are present in the solution.
To which electrodes are the ions attracted during this electrolysis?\[
\begin{array}{|c|c|c|}
\hline & \text { attracted to anode } & \text { attracted to cathode } \\
\hline \text { A } & \mathrm{Cu}^{2+} \text { and } \mathrm{H}^{+} & \mathrm{OH}^{-} \text {and } \mathrm{SO}_4{ }^{2-} \\
\text { B } & \mathrm{Cu}^{2+} \text { and } \mathrm{SO}_4^{2-} & \mathrm{H}^{+} \text {and } \mathrm{OH}^{-} \\
\text {C } & \mathrm{H}^{+} \text {and } \mathrm{OH}^{-} & \mathrm{Cu}^{2+} \text { and } \mathrm{SO}_4^{2-} \\
\text { D } & \mathrm{OH}^{-} \text {and } \mathrm{SO}_4^{2-} & \mathrm{Cu}^{2+} \text { and } \mathrm{H}^{+} \\
\hline
\end{array}
\]CorrectIncorrect
0 of 6 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 6 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) The diagram shows apparatus for plating a spoon with silver. Which metal could not be used for electroplating by using an aqueous solution? Which apparatus could be used to electroplate an iron nail with copper? The diagram shows a method used to copper-plate a pan Which apparatus is used to plate a nickel object with silver? Iron can be electroplated with zinc to make it resistant to corrosion. Which row about electroplating iron with zinc is correct? \[
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
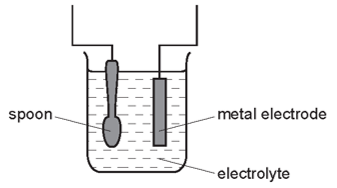 Which statement is not correct?
Which statement is not correct?
2. Question
1 point(s)
3. Question
1 point(s)
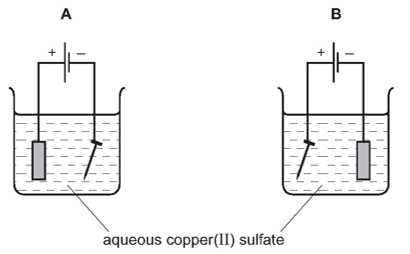
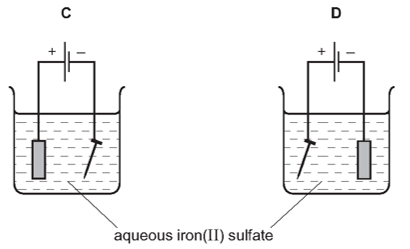
4. Question
1 point(s)
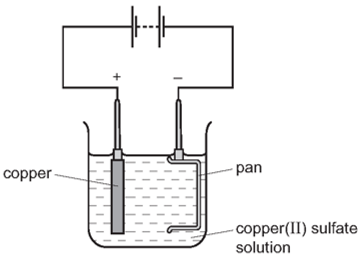 Which equation represents the reaction at the cathode?
Which equation represents the reaction at the cathode?
5. Question
1 point(s)
6. Question
1 point(s)
\begin{array}{|c|c|c|c|}
\hline & \begin{array}{c}
\text { positive electrode } \\
\text { (anode) }
\end{array} & \begin{array}{c}
\text { negative electrode } \\
\text { (cathode) }
\end{array} & \text { electrolyte } \\
\hline \text { A } & \text { iron } & \text { zinc } & \text { iron nitrate } \\
\text { B } & \text { iron } & \text { zinc } & \text { zinc nitrate } \\
\text { C } & \text { zinc } & \text { iron } & \text { iron nitrate } \\
\text { D } & \text { zinc } & \text { iron } & \text { zinc nitrate } \\
\hline
\end{array}
\]
