0 of 12 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 12 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) A liquid at room temperature fills a flask and a glass tube to level \(X\). An engineer wants to fix a steel washer on to a steel rod. The rod is just too big to fit into the hole of the washer. The diagram shows electricity cables being put up on a warm day. The cables are left loose between the poles, as shown in the diagram. A circular metal disc is heated. A telephone engineer connects a wire between two poles when the weather is very cold. \[ A long thin bar of copper is heated evenly along its length. A solid and a gas are each given the same increase in temperature. The gas is kept at a constant pressure. Which row is correct? A hole is drilled in a metal plate. What happens to the length of the plate and to the diameter of the hole when the plate is cooled? \[ Why are small gaps left between the metal rails of a railway track? Equal volumes of solids and liquids experience different changes of volume when they are heated through the same temperature range. What is the reason for this? A bimetallic strip is used to control the temperature of electrical appliances. It is made of two different metals fixed together. When a bridge is built, a gap is left between each concrete slab. Why are these gaps left?
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
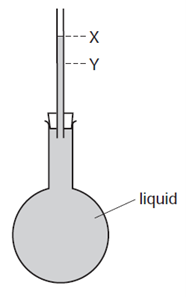
The flask is now placed in ice, and the liquid level in the tube falls to level \(Y\).
Why does the level fall?
2. Question
1 point(s)
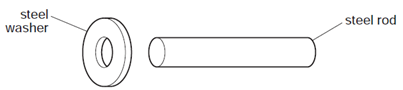
How can the engineer fit the washer on to the rod?
3. Question
1 point(s)
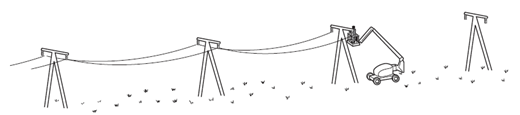
Why are the cables left loose?
4. Question
1 point(s)
Which quantity decreases?
5. Question
1 point(s)
He makes the wire very loose. The wire passes over a road.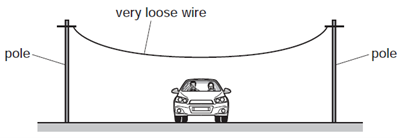
The weather changes and it becomes very hot.
What could happen to the wire and why?
\begin{array}{|c|c|c|}
\hline & \text { what could happen } & \text { why } \\
\hline \text { A } & \text { it breaks } & \text { it contracts } \\
\text { B } & \text { it breaks } & \text { it expands } \\
\text { C } & \begin{array}{c}
\text { it sags and touches } \\
\text { cars on the road }
\end{array} & \text { it contracts } \\
\text { D } & \begin{array}{c}
\text { it sags and touches } \\
\text { cars on the road }
\end{array} & \text { it expands } \\
\hline
\end{array}
\]
6. Question
1 point(s)
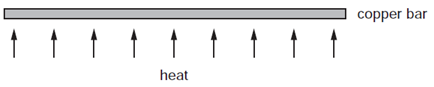
What happens to the bar?
7. Question
1 point(s)
\[
\begin{array}{|c|c|c|}
\hline & \begin{array}{l}
\text { the one which } \\
\text { expands most }
\end{array} & \text { the reason } \\
\hline \text { A } & \text { the gas } & \begin{array}{l}
\text { molecules in the gas each expand } \\
\text { more than the solid molecules }
\end{array} \\
\hline \text { B } & \text { the gas } & \begin{array}{l}
\text { the molecules in the solid } \\
\text { are held strongly together }
\end{array} \\
\hline \text { C } & \text { the solid } & \begin{array}{l}
\text { molecules in the solid each expand } \\
\text { more than the gas molecules }
\end{array} \\
\hline \text { D } & \text { the solid } & \begin{array}{l}
\text { all the molecules in the gas are } \\
\text { separate from one another }
\end{array} \\
\hline
\end{array}
\]
8. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { length of plate } & \text { diameter of hole } \\
\hline \text { A } & \text { decreases } & \text { decreases } \\
\text { B } & \text { decreases } & \text { increases } \\
\text { C } & \text { increases } & \text { decreases } \\
\text { D } & \text { increases } & \text { increases } \\
\hline
\end{array}
\]
9. Question
1 point(s)
10. Question
1 point(s)
11. Question
1 point(s)
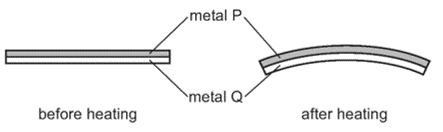 The diagram shows the shape of the bimetallic strip before and after heating. Which statement is correct?
The diagram shows the shape of the bimetallic strip before and after heating. Which statement is correct?
12. Question
1 point(s)
0 of 10 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Two metal blocks \(\mathrm{X}\) and \(\mathrm{Y}\) are at room temperature. Each block is heated so that its temperature rises by \(10^{\circ} \mathrm{C}\). \[ The thermal capacity of solid \(\mathrm{Y}\) is greater than that of solid \(\mathrm{Z}\). The same quantity of thermal (heat) energy is given to two objects \(X\) and \(Y\). The temperature rise of object \(X\) is less than the temperature rise of object \(Y\). A block of copper and a block of lead are heated. The internal energy of each block increases by the same amount. The block of copper has a lower thermal capacity than the block of lead. Which conclusion can be made from this information? Which quantity gives the thermal capacity of a beaker? A block of aluminium of mass 2.0kg has an initial temperature of 20°C. It absorbs 7300J of thermal energy. What is the final temperature? A student calculates the energy needed to raise the temperature of an aluminium block from 50°C to 60°C. Which statement about the measured energy is correct? An aluminium block has a mass of 200g. The specific heat capacity of aluminium is 900J/(kg°C). How much energy is needed to increase the temperature of the block from 20°C to 110°C? A block of metal absorbs \(2000 J\) of thermal energy. Four blocks are made from different metals. Each block is heated for five minutes with an identical heater. Assume there is no energy loss from the blocks. \[
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
The blocks are now allowed to cool back to room temperature.
Block \(Y\) has a greater thermal capacity than block \(X\).
Which block needs more thermal (heat) energy to heat it up by \(10^{\circ} \mathrm{C}\) and which block loses more thermal (heat) energy as it cools back to room temperature?
\begin{array}{|c|c|c|}
\hline {} & {\text { more energy }} \\
& \text { heating } & \text { cooling } \\
\hline \text { A } & \mathrm{X} & \mathrm{X} \\
\text { B } & \mathrm{X} & \mathrm{Y} \\
\text { C } & \mathrm{Y} & \mathrm{X} \\
\text { D } & \mathrm{Y} & \mathrm{Y} \\
\hline
\end{array}
\]
2. Question
1 point(s)
What is a consequence of this?
3. Question
1 point(s)
What accounts for this difference?
4. Question
1 point(s)
5. Question
1 point(s)
6. Question
1 point(s)
7. Question
1 point(s)
8. Question
1 point(s)
9. Question
1 point(s)
The temperature of the block rises from \(10^{\circ} C\) to \(20^{\circ} C\). The mass of the block is \(2.0 kg\). What is the specific heat capacity of the metal?
10. Question
1 point(s)
The table gives the masses of the blocks and the temperature rises. Which metal has the highest specific heat capacity?
\begin{array}{|c|c|c|}
\hline & \text { mass of block } / kg & \text { temperature rise } /{ }^{\circ} C \\
\hline \text { A } & 2.0 & 5.0 \\
\text { B } & 2.0 & 9.0 \\
\text { C } & 4.0 & 5.0 \\
\text { D } & 4.0 & 9.0 \\
\hline
\end{array}
\]
