0 of 13 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 13 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Which structure is incorrect? Which statement is correct? Which fuel could be gasoline? What is not the correct use of the fraction named? \[ Fuel oil and naphtha are two fractions obtained from petroleum. \[ Fuel oil, gasoline, kerosene and naphtha are four fractions obtained from the fractional distillation of petroleum. \[ Which list shows the fractions obtained from distilling petroleum, in order of increasing boiling point? The diagram shows the separation of petroleum into fractions. \[ Which row shows the correct use of a fraction obtained by the fractional distillation of petroleum? \[ Which statement about petroleum is not correct? The table shows the composition of four different types of petroleum (crude oil). \[ Which type of petroleum is best for the motor vehicle industry? The table shows some fractions that are obtained from petroleum by fractional distillation, together with some of their uses. \[ Bitumen is a substance obtained from the fractional distillation of petroleum. \[
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
3. Question
1 point(s)
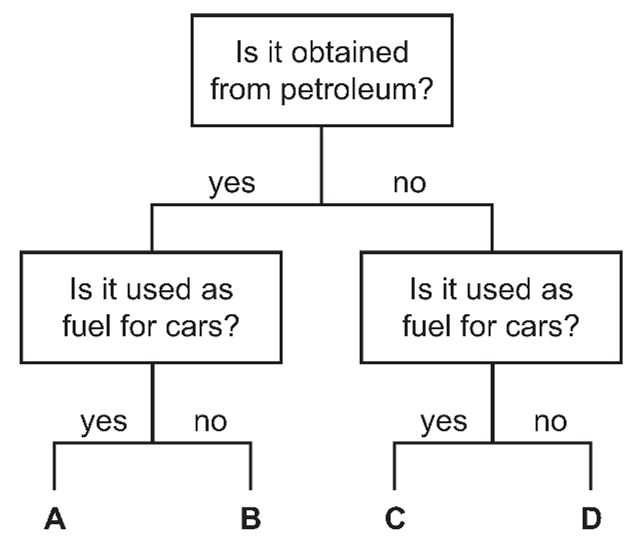
4. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { name of fraction } & \text { use } \\
\hline \text { A } & \text { fuel oil } & \text { making waxes } \\
\text { B } & \text { gas oil } & \text { fuel in diesel engines } \\
\text { C } & \text { kerosene } & \text { jet fuel } \\
\text { D } & \text { naphtha } & \text { making chemicals } \\
\hline
\end{array}
\]
5. Question
1 point(s)
What are the major uses of these fractions?
\begin{array}{|c|c|c|}
\hline & \text { fuel oil } & \text { naphtha } \\
\hline \text { A } & \text { jet fuel } & \text { making chemicals } \\
\text { B } & \text { jet fuel } & \text { making roads } \\
\text { C } & \text { ship fuel } & \text { making chemicals } \\
\text { D } & \text { ship fuel } & \text { making roads } \\
\hline
\end{array}
\]
6. Question
1 point(s)
What is the order of the boiling points of these fractions?
\begin{array}{|c|l|}
\hline & \text { highest boiling point } \rightarrow \text { lowest boiling point } \\
\hline \text { A } & \text { fuel oil } \rightarrow \text { kerosene } \rightarrow \text { gasoline } \rightarrow \text { naphtha } \\
\text { B } & \text { fuel oil } \rightarrow \text { kerosene } \rightarrow \text { naphtha } \rightarrow \text { gasoline } \\
\text { C } & \text { gasoline } \rightarrow \text { naphtha } \rightarrow \text { kerosene } \rightarrow \text { fuel oil } \\
\text { D } & \text { naphtha } \rightarrow \text { gasoline } \rightarrow \text { kerosene } \rightarrow \text { fuel oil } \\
\hline
\end{array}
\]
7. Question
1 point(s)
8. Question
1 point(s)
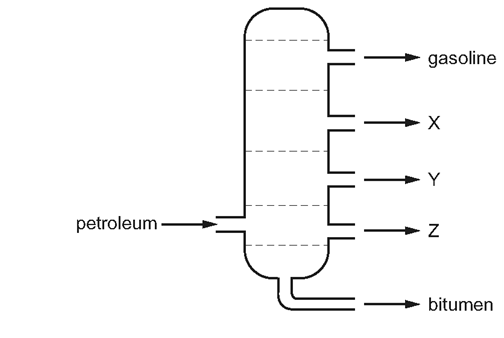
What could \(X, Y\) and \(Z\) represent?
\begin{array}{|c|c|c|c|}
\hline & \text { X } & Y & Z \\
\hline \text { A } & \text { diesel oil } & \text { lubricating fraction } & \text { paraffin } \\
\text { B } & \text { lubricating fraction } & \text { diesel oil } & \text { paraffin } \\
\text { C } & \text { paraffin } & \text { lubricating fraction } & \text { diesel oil } \\
\text { D } & \text { paraffin } & \text { diesel oil } & \text { lubricating fraction } \\
\hline
\end{array}
\]
9. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { fraction } & \text { use } \\
\hline \text { A } & \text { bitumen } & \text { making waxes and polishes } \\
\text { B } & \text { fuel oil } & \text { aircraft fuel } \\
\text { C } & \text { kerosene } & \text { fuel for ships } \\
\text { D } & \text { naphtha } & \text { making chemicals } \\
\hline
\end{array}
\]
10. Question
1 point(s)
11. Question
1 point(s)
\begin{array}{|l|c|c|c|c|}
\hline {\text { fraction }} & \begin{array}{c}
\text { Arabian Heavy } \\
/ \%
\end{array} & \begin{array}{c}
\text { Arabian Light } \\
/ \%
\end{array} & \begin{array}{c}
\text { Iranian Heavy } \\
/ \%
\end{array} & \begin{array}{c}
\text { North Sea } \\
/ \%
\end{array} \\
\hline \text { gasoline } & 18 & 21 & 21 & 23 \\
\text { kerosene } & 11.5 & 13 & 13 & 15 \\
\text { diesel oil } & 18 & 20 & 20 & 24 \\
\text { fuel oil } & 52.5 & 46 & 46 & 38 \\
\hline
\end{array}
\]
12. Question
1 point(s)
\[
\begin{array}{|c|c|}
\hline \text { fraction } & \text { use } \\
\hline \text { refinery gas } & \text { cooking } \\
\text { gasoline } & \text { fuel for cars } \\
1 & \text { making chemicals } \\
2 & \text { jet fuel } \\
3 & \text { fuel for ships } \\
\text { bitumen } & \text { making roads } \\
\hline
\end{array}
\]
Which row correctly identifies fractions 1,2 and 3 ?
\begin{array}{|c|c|c|c|}
\hline & 1 & 2 & 3 \\
\hline \text { A } & \text { diesel oil } & \text { fuel oil } & \text { lubricating fraction } \\
\text { B } & \text { fuel oil } & \text { diesel oil } & \text { kerosene } \\
\text { C } & \text { kerosene } & \text { naphtha } & \text { diesel oil } \\
\text { D } & \text { naphtha } & \text { kerosene } & \text { fuel oil } \\
\hline
\end{array}
\]
13. Question
1 point(s)
Which row describes its boiling point and the size of its molecules?
\begin{array}{|c|c|c|}
\hline & \text { boiling point } & \text { size of molecules } \\
\hline \text { A } & \text { high } & \text { large } \\
\text { B } & \text { high } & \text { small } \\
\text { C } & \text { low } & \text { large } \\
\text { D } & \text { low } & \text { small } \\
\hline
\end{array}
\]
0 of 22 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 22 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) The structures of three compounds are shown. Which structures show compounds that are members of the same homologous series? The diagram shows the structure of a compound. \[ The diagram shows the structures of three compounds. Which pair of compounds are members of the same homologous series? The table shows bonds that are present and bonds that are not present in compound X. \[ What type of compound is X? Which structure is correctly named? Which structure shows a carboxylic acid? Alkenes have the general formula \(\mathrm{C}_n \mathrm{H}_{2 n}\). Which of the following is an alkene? Which group of compounds is part of a homologous series? Which statement about a homologous series is correct? Which statements about homologous series are correct? Which two compounds are molecules which both contain a double bond? Methane, ethane and propane belong to a family of hydrocarbons called alkanes. \(X, Y\) and \(Z\) are three hydrocarbons. Which compound does not belong to the same homologous series as the other three compounds? The structures of four compounds are shown. Which are members of the same homologous series? Ethene, propene and butene are all members of the same homologous series. The list gives the names of four organic compounds. ethane Which bond do all four compounds contain? Which group of compounds is part of a homologous series? PVA is a polymer. The monomer has the structure shown. \[ The diagram shows the structures of three compounds.
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
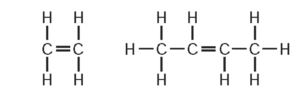
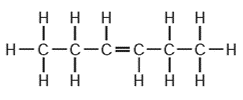 Why do these substances all belong to the same homologous series?
Why do these substances all belong to the same homologous series?
2. Question
1 point(s)
3. Question
1 point(s)
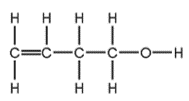 To which classes of compound does this molecule belong?
To which classes of compound does this molecule belong?
\begin{array}{|c|c|c|c|}
\hline & \text { alkane } & \text { alkene } & \text { alcohol } \\
\hline \text { A } & \text { no } & \text { no } & \text { no } \\
\text { B } & \text { no } & \text { yes } & \text { yes } \\
\text { C } & \text { yes } & \text { no } & \text { yes } \\
\text { D } & \text { yes } & \text { yes } & \text { yes } \\
\hline
\end{array}
\]
4. Question
1 point(s)
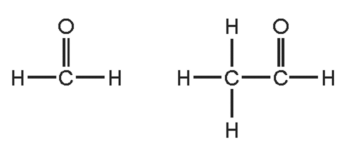
 Why do these three compounds belong to the same homologous series?
Why do these three compounds belong to the same homologous series?
5. Question
1 point(s)
6. Question
1 point(s)
\begin{array}{|c|c|}
\hline \text { bond } & \\
\hline \mathrm{C}-\mathrm{C} & \checkmark \\
\mathrm{C}=\mathrm{C} & ✘ \\
\mathrm{C}-\mathrm{H} & \checkmark \\
\mathrm{C}-\mathrm{O} & \checkmark \\
\mathrm{C}=\mathrm{O} & \checkmark \\
\mathrm{O}-\mathrm{H} & \checkmark \\
\hline
\end{array}
\]
7. Question
1 point(s)
8. Question
1 point(s)
9. Question
1 point(s)
10. Question
1 point(s)
11. Question
1 point(s)
12. Question
1 point(s)
1 All members have similar chemical properties.
2 All members have the same molecular mass.
3 Ethane and ethene are members of the same homologous series.
4 Ethane and propane are members of the same homologous series.
13. Question
1 point(s)
14. Question
1 point(s)
What is the general formula of an alkane?
15. Question
1 point(s)
\[
\mathrm{X}\quad\mathrm{CH}_2=\mathrm{CH}_2 \quad\quad\quad \mathrm{Y} \quad\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2 \quad\quad\quad \mathrm{Z} \quad \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}=\mathrm{CH}_2
\]
What do compounds \(X, Y\) and \(Z\) have in common?
1 They are all alkenes.
2 They are all part of the same homologous series.
3 They all have the same boiling point.
16. Question
1 point(s)
17. Question
1 point(s)
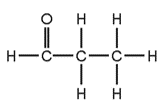
18. Question
1 point(s)
Which statement explains why ethene, propene and butene have similar chemical properties?
19. Question
1 point(s)
ethanoic acid
ethanol
ethene
20. Question
1 point(s)
21. Question
1 point(s)
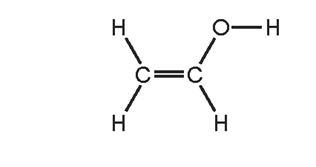
To which homologous series does this compound belong?
\begin{array}{|c|c|c|}
\hline & \text { alcohols } & \text { alkenes } \\
\hline \text { A } & \checkmark & \checkmark \\
\text { B } & \checkmark & x \\
\text { C } & x & \checkmark \\
\text { D } & x & x \\
\hline
\end{array}
\]
22. Question
1 point(s)
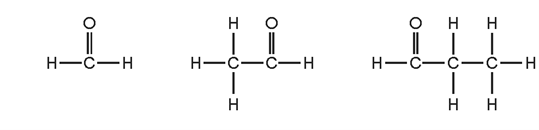
Why do these three compounds belong to the same homologous series?
0 of 8 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 8 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Which products are obtained by the cracking of an alkane? \[ Which type of reaction takes place when methane reacts with chlorine in the presence of ultraviolet light? Which equation represents the incomplete combustion of propane, \(\mathrm{C}_3 \mathrm{H}_8\) ? Butane and methylpropane are isomers with molecular formula \(\mathrm{C}_4 \mathrm{H}_{10}\). 1 They have similar chemical properties. Butane reacts as shown. The main constituent of natural gas is hydrocarbon \(X\). \[ Alkenes are manufactured by cracking hydrocarbons obtained from petroleum. \[ Which equation represents incomplete combustion of ethane?
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
\begin{array}{|c|c|c|c|}
\hline & \text { alkene } & \text { hydrogen } & \text { water } \\
\hline \text { A } & \checkmark & \checkmark & \checkmark \\
\text { B } & \checkmark & \checkmark & x \\
\text { C } & \checkmark & x & \checkmark \\
\text { D } & x & \checkmark & \checkmark \\
\hline
\end{array}
\]
2. Question
1 point(s)
3. Question
1 point(s)
4. Question
1 point(s)
Which statements are correct?
2 They have the same general formula.
3 They have the same structural formula.
5. Question
1 point(s)
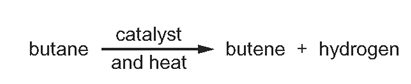
What is this type of reaction?
6. Question
1 point(s)
To which homologous series does \(X\) belong and how many atoms are in one molecule of \(X\) ?
\begin{array}{|c|c|c|}
\hline & \text { homologous series } & \begin{array}{c}
\text { number of atoms } \\
\text { in one molecule }
\end{array} \\
\hline \text { A } & \text { alkane } & 1 \\
\text { B } & \text { alkane } & 5 \\
\text { C } & \text { alkene } & 1 \\
\text { D } & \text { alkene } & 5 \\
\hline
\end{array}
\]
7. Question
1 point(s)
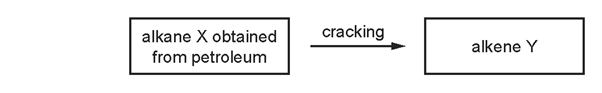
Which row describes the process of cracking?
\begin{array}{|c|c|c|c|c|}
\hline & \begin{array}{c}
\text { size of } X \\
\text { molecules }
\end{array} & \begin{array}{c}
\text { size of } Y \\
\text { molecules }
\end{array} & \begin{array}{c}
\text { catalyst } \\
\text { required }
\end{array} & \begin{array}{c}
\text { temperature } \\
\text { required }
\end{array} \\
\hline \text { A } & \text { large } & \text { small } & \text { no } & \text { low } \\
\text { B } & \text { large } & \text { small } & \text { yes } & \text { high } \\
\text { C } & \text { small } & \text { large } & \text { no } & \text { low } \\
\text { D } & \text { small } & \text { large } & \text { yes } & \text { high } \\
\hline
\end{array}
\]
8. Question
1 point(s)
0 of 9 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 9 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) A compound has the formula \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}=\mathrm{CH}_2\). \[ Which product is obtained when bromine reacts with propene, \(\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2\) ? Alkanes undergo substitution reactions with chlorine in the presence of ultraviolet light. Increasing the number of atoms in one molecule of a hydrocarbon increases the amount of energy released when it burns. What is the correct order? During the process of cracking hydrocarbons, an ………. 1 ……….. is converted into an ……….. 2 ……….. . Which words complete gaps \(1,2,3\) and \(4\)? \[ A hydrocarbon A is cracked to make B and hydrogen. \[ The equation shows an industrial process. What is the name of compound X? X, Y and Z are three hydrocarbons. 1 They are all alkenes. The diagram shows a flow diagram. \[
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
Which row in the table shows the type of compound and the colour change when aqueous bromine is added?
\begin{array}{|c|c|c|}
\hline & \text { type of compound } & \text { colour change } \\
\hline \text { A } & \text { saturated } & \text { brown to colourless } \\
\text { B } & \text { saturated } & \text { colourless to brown } \\
\text { C } & \text { unsaturated } & \text { brown to colourless } \\
\text { D } & \text { unsaturated } & \text { colourless to brown } \\
\hline
\end{array}
\]
2. Question
1 point(s)
3. Question
1 point(s)
Which equation shows a reaction of this type?
4. Question
1 point(s)
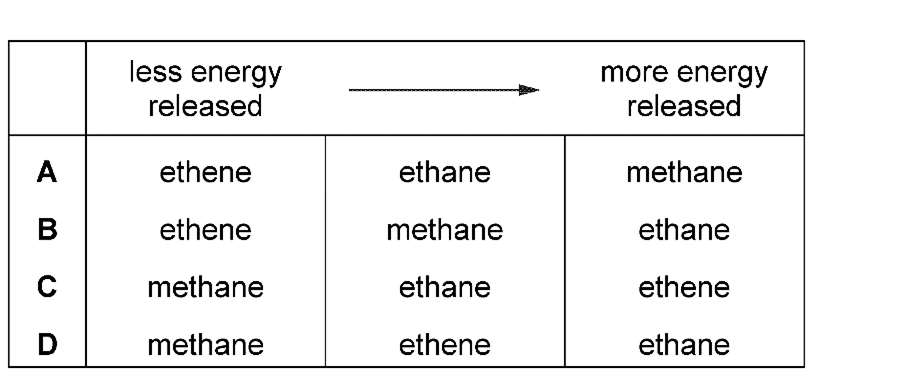
5. Question
1 point(s)
The presence of an ……….. 3 ………. can be shown by a visible reaction with ……….. 4 ………. .
\begin{array}{|c|c|c|c|c|}
\hline & 1 & 2 & 3 & 4 \\
\hline \text { A } & \text { alkane } & \text { alkene } & \text { alkene } & \text { bromine } \\
\text { B } & \text { alkane } & \text { alkene } & \text { alkene } & \text { steam } \\
\text { C } & \text { alkene } & \text { alkane } & \text { alkane } & \text { bromine } \\
\text { D } & \text { alkene } & \text { alkane } & \text { alkane } & \text { steam } \\
\hline
\end{array}
\]
6. Question
1 point(s)
Compound C is formed by the addition polymerisation of B.
To which homologous series do A, B and C belong?
\begin{array}{|c|c|c|}
\hline & \text { alkene } & \text { alkane } \\
\hline \text { A } & \text { A } & \text { B and C } \\
\text { B } & \text { B } & \text { A and C } \\
\text { C } & \text { C } & \text { A and B } \\
\text { D } & – & \text { A and C } \\
\hline
\end{array}
\]
7. Question
1 point(s)

8. Question
1 point(s)
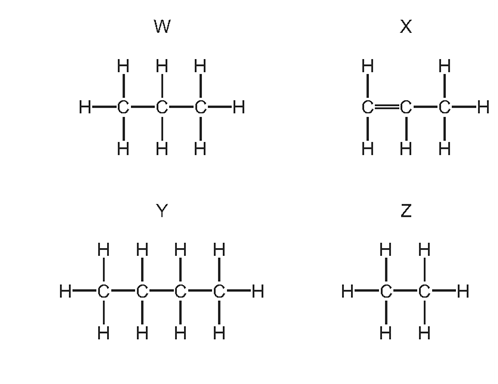
![]()
What do compounds X, Y and Z have in common?
2 They are all part of the same homologous series.
3 They all have the same boiling point.
9. Question
1 point(s)
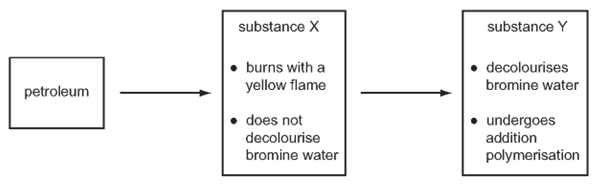
Which type of organic compounds are X and Y ?
\begin{array}{|l|c|l|}
\hline & \text { substance } X & \text { substance } Y \\
\hline \text { A } & \text { alcohol } & \text { alkane } \\
\text { B } & \text { alkane } & \text { alkene } \\
\text { C } & \text { alkene } & \text { alkane } \\
\text { D } & \text { alkane } & \text { alcohol } \\
\hline
\end{array}
\]
0 of 12 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 12 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) What is not essential for the formation of ethanol by fermentation? Propanol is oxidised by acidified potassium manganate(VII) in a similar way to ethanol. Ethanol is made by fermentation of sugars and by the catalytic addition of steam to ethene. Ethanol can be formed by: 1 fermentation Which of these processes use a catalyst? \[ Which row describes an advantage and a disadvantage of making ethanol by fermentation? \[ The diagram shows a reaction sequence. \[ The structure of compound R is shown. Substance \(Z\) has the following characteristics. 1 It burns in an excess of oxygen to form carbon dioxide and water. What is substance \(Z\) ? Ethanol is manufactured from petroleum by reacting ethene with steam. 1 Ethene is obtained from the cracking of alkanes. The diagram shows a reaction sequence. \[ The table shows the boiling points of four members of the homologous series of alcohols. Sugar can be fermented to produce ethanol. 1 Leave in a warm place. What is the correct order of these stages?
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
Which compound is produced by the oxidation of propanol with acidified potassium manganate(VII)?
3. Question
1 point(s)
What are two advantages of making ethanol by the catalytic addition of steam to ethene rather than by fermentation of sugars?
4. Question
1 point(s)
2 reaction between steam and ethene.
\begin{array}{|c|c|c|}
\hline & 1 & 2 \\
\hline \text { A } & \checkmark & \checkmark \\
\text { B } & \checkmark & x \\
\text { C } & x & \checkmark \\
\text { D } & x & x \\
\hline
\end{array}
\]
5. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { advantage } & \text { disadvantage } \\
\hline \text { A } & \text { uses a renewable resource } & \text { occurs at a slow rate } \\
\text { B } & \text { needs a high temperature } & \text { produces impure ethanol as a product } \\
\text { C } & \text { produces pure ethanol as a product } & \text { needs a high temperature } \\
\text { D } & \text { occurs at a slow rate } & \text { uses a non-renewable resource } \\
\hline
\end{array}
\]
6. Question
1 point(s)
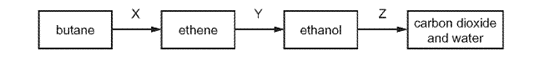
Which row names the processes X, Y and Z ?
\begin{array}{|c|c|c|c|}
\hline & \mathrm{X} & \mathrm{Y} & \mathrm{Z} \\
\hline \text { A } & \text { cracking } & \text { fermentation } & \text { respiration } \\
\text { B } & \text { cracking } & \text { hydration } & \text { combustion } \\
\text { C } & \text { distillation } & \text { fermentation } & \text { respiration } \\
\text { D } & \text { distillation } & \text { hydration } & \text { combustion } \\
\hline
\end{array}
\]
7. Question
1 point(s)
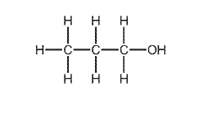
What is R?
8. Question
1 point(s)
2 It is oxidised by air to form a liquid smelling of vinegar.
3 It reacts with carboxylic acids to form esters.
9. Question
1 point(s)
Which statements about this process are correct?
2 The process is carried out in the presence of yeast.
3 The reaction is an addition reaction.
4 The rate of reaction is increased by a catalyst.
10. Question
1 point(s)
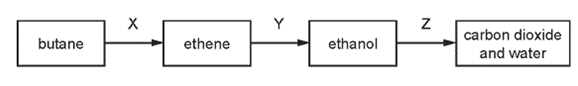
Which row names the processes X, Y and Z ?
\begin{array}{|c|c|c|c|}
\hline & \mathrm{X} & \mathrm{Y} & Z \\
\hline \text { A } & \text { cracking } & \text { fermentation } & \text { respiration } \\
\text { B } & \text { cracking } & \text { hydration } & \text { combustion } \\
\text { C } & \text { distillation } & \text { fermentation } & \text { respiration } \\
\text { D } & \text { distillation } & \text { hydration } & \text { combustion } \\
\hline
\end{array}
\]
11. Question
1 point(s)
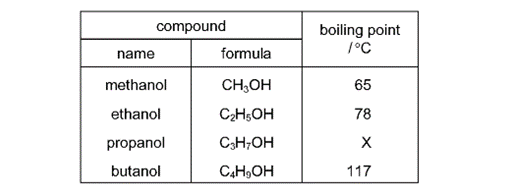
What is the value of X ?
12. Question
1 point(s)
Some of the stages in the process to produce and purify ethanol are listed.
2 Add yeast.
3 Fractionally distil the solution.
4 Dissolve the sugar in water.
5 Filter to remove the yeast.
6 Crush some sugar cane.
0 of 10 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Which statement about aqueous ethanoic acid is correct? Which statement about ethanoic acid is correct? Which statement describes the compound shown below? Molecule \(X\) is both an alkene and a carboxylic acid. \[ Which structure shows a carboxylic acid? Which esters have the molecular formula \(\mathrm{C}_5 \mathrm{H}_{10} \mathrm{O}_2\) ? 1 ethyl propanoate The structure of an ester is shown. \[ The structure of an alkene and the structure of an ester are shown. \[ The structure of ester W is shown. \[ The structure of an ester is shown. \[
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
3. Question
1 point(s)
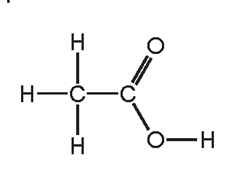
4. Question
1 point(s)
Which row describes \(X ?\)
\begin{array}{|c|c|c|}
\hline & \text { saturated } & -\mathrm{COOH} \text { present } \\
\hline \text { A } & \text { no } & \text { no } \\
\text { B } & \text { no } & \text { yes } \\
\text { C } & \text { yes } & \text { no } \\
\text { D } & \text { yes } & \text { yes } \\
\hline
\end{array}
\]
5. Question
1 point(s)
6. Question
1 point(s)
2 propyl ethanoate
3 butyl methanoate
4 methyl butanoate
7. Question
1 point(s)
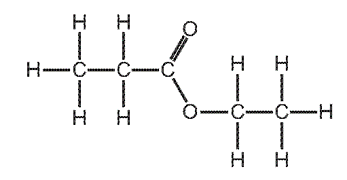
Which alcohol and carboxylic acid produce this ester?
\begin{array}{|c|c|c|}
\hline & \text { alcohol } & \text { carboxylic acid } \\
\hline \text { A } & \text { ethanol } & \text { ethanoic acid } \\
\text { B } & \text { ethanol } & \text { propanoic acid } \\
\text { C } & \text { propanol } & \text { ethanoic acid } \\
\text { D } & \text { propanol } & \text { propanoic acid } \\
\hline
\end{array}
\]
8. Question
1 point(s)
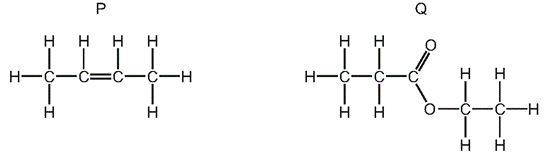
What are the names of P and Q?
\begin{array}{|c|c|c|}
\hline & \mathrm{P} & \mathrm{Q} \\
\hline \text { A } & \text { but-1-ene } & \text { ethyl propanoate } \\
\text { B } & \text { but-1-ene } & \text { propyl ethanoate } \\
\text { C } & \text { but-2-ene } & \text { ethyl propanoate } \\
\text { D } & \text { but-2-ene } & \text { propyl ethanoate } \\
\hline
\end{array}
\]
9. Question
1 point(s)
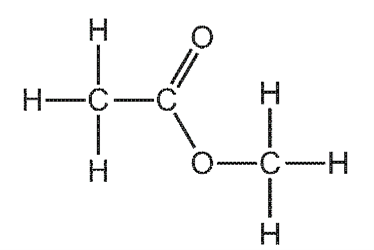
Which row gives the names of ester W and the carboxylic acid and alcohol from which it is made?
\begin{array}{|c|c|c|c|}
\hline & \text { name of ester W } & \text { carboxylic acid } & \text { alcohol } \\
\hline \text { A } & \text { ethyl methanoate } & \text { ethanoic acid } & \text { methanol } \\
\text { B } & \text { ethyl methanoate } & \text { methanoic acid } & \text { ethanol } \\
\text { C } & \text { methyl ethanoate } & \text { ethanoic acid } & \text { methanol } \\
\text { D } & \text { methyl ethanoate } & \text { methanoic acid } & \text { ethanol } \\
\hline
\end{array}
\]
10. Question
1 point(s)
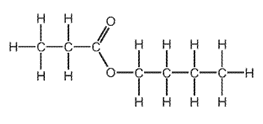
Which combination of carboxylic acid and alcohol produces this ester?
\begin{array}{|c|c|c|}
\hline & \text { carboxylic acid } & \text { alcohol } \\
\hline \text { A } & \text { butanoic acid } & \text { ethanol } \\
\text { B } & \text { butanoic acid } & \text { propanol } \\
\text { C } & \text { ethanoic acid } & \text { butanol } \\
\text { D } & \text { propanoic acid } & \text { butanol } \\
\hline
\end{array}
\]
0 of 8 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 8 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Which row describes the formation of a polymer? \[ Which equation represents the formation of poly(propene) from propene? The diagram shows the structure of a monomer and of the polymer made from it. \[ A section of a polymer is shown. The structure of a polymer is shown. A polymer linkage contains carbon, hydrogen, nitrogen and oxygen atoms. \[ The equation shows the formation of a polymer called Kevlar. \[ The diagram shows part of the molecule of a polymer.
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
\begin{array}{|c|c|c|}
\hline & \text { monomer } & \text { polymer } \\
\hline \text { A } & \text { ethane } & \text { poly(ethane) } \\
\text { B } & \text { ethane } & \text { poly(ethene) } \\
\text { C } & \text { ethene } & \text { poly(ethane) } \\
\text { D } & \text { ethene } & \text { poly(ethene) } \\
\hline
\end{array}
\]
2. Question
1 point(s)
3. Question
1 point(s)
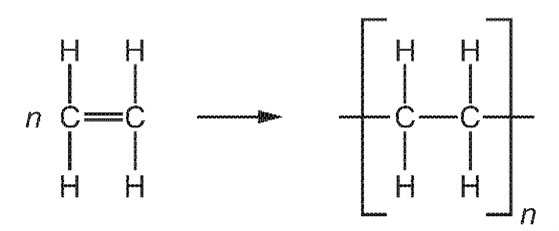
What are the monomer and polymer?
\begin{array}{|c|c|c|}
\hline & \text { monomer } & \text { polymer } \\
\hline \text { A } & \text { ethane } & \text { poly(ethane) } \\
\text { B } & \text { ethane } & \text { poly(ethene) } \\
\text { C } & \text { ethene } & \text { poly(ethane) } \\
\text { D } & \text { ethene } & \text { poly(ethene) } \\
\hline
\end{array}
\]
4. Question
1 point(s)
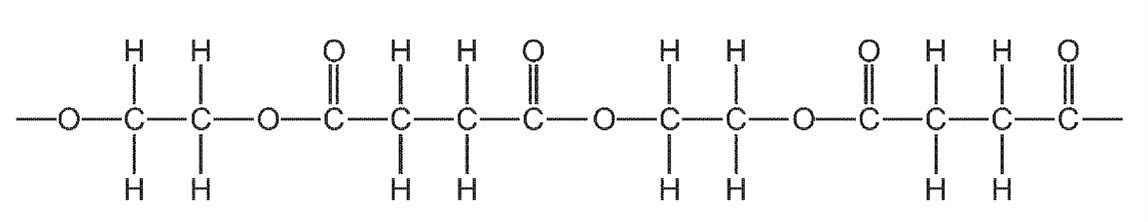
How many different types of monomer units formed this section of polymer?
5. Question
1 point(s)
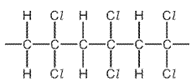
Which monomer is used to make this polymer?
6. Question
1 point(s)
Which row about the polymer is correct?
\begin{array}{|c|c|c|}
\hline & \begin{array}{c}
\text { type of } \\
\text { polymer }
\end{array} & \text { formed by } \\
\hline \text { A } & \text { polyamide } & \text { addition polymerisation } \\
\text { B } & \text { polyamide } & \text { condensation polymerisation } \\
\text { C } & \text { polyester } & \text { addition polymerisation } \\
\text { D } & \text { polyester } & \text { condensation polymerisation } \\
\hline
\end{array}
\]
7. Question
1 point(s)
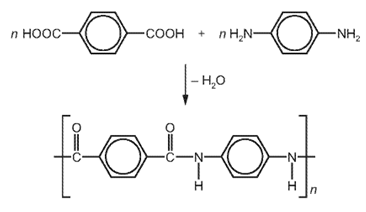
Which row describes Kevlar?
\begin{array}{|c|c|c|}
\hline & \text { how the polymer is formed } & \text { type of polymer } \\
\hline \text { A } & \text { addition polymerisation } & \text { polyamide } \\
\text { B } & \text { addition polymerisation } & \text { polyester } \\
\text { C } & \text { condensation polymerisation } & \text { polyamide } \\
\text { D } & \text { condensation polymerisation } & \text { polyester } \\
\hline
\end{array}
\]
8. Question
1 point(s)
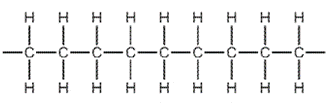
Which diagram shows the monomer from which this polymer could be manufactured?
