0 of 15 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) How many neutrons are in a nucleus of the nuclide \({ }_{17}^{37} \mathrm{Cl}\) ? A certain element has several isotopes. A neutral atom consists of electrons orbiting a nucleus. The nucleus contains protons and neutrons. Below are the symbols for five different nuclides. A proton and a neutron are each close to a positive nucleus. How does the charge on the nucleus affect the proton and the neutron, if at all? Below are four statements about isotopes of a certain element. A nucleus of element \(X\) is represented as \({ }_{26}^{56} X\). The charge on a proton is \(e\). \[ A particular nuclide has the symbol \({ }_{17}^{37} \mathrm{Cl}\). \({ }_6^{14} \mathrm{C}\) is a nuclide of carbon. \[ A nuclide has the symbol \({ }_{10}^{22} \mathrm{Ne}\). The nucleus of an americium atom contains 146 neutrons and 95 protons. It decays by emitting an \(\alpha\)-particle. How many neutrons and how many protons remain in the nucleus when this form of americium decays? \[ Which statement is correct for the nucleus of any atom? Which particles are emitted during thermionic emission? A uranium \({ }_{92}^{238} \mathrm{U}\) nudeus emits an \(\alpha\)-particle. \[
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
Which statement about these isotopes is correct?
3. Question
1 point(s)
Which statement about the atom must be correct?
4. Question
1 point(s)
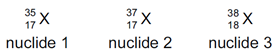

Which two nuclides are isotopes of the same element?
5. Question
1 point(s)

6. Question
1 point(s)
Which statement about the isotopes must be correct?
7. Question
1 point(s)
Which is an isotope of element \(X\) ?
8. Question
1 point(s)
What is the charge on an electron and what is the charge on a neutron?
\begin{array}{|c|c|c|}
\hline & \text { electron } & \text { neutron } \\
\hline \text { A } & e & e \\
\text { B } & e & 0 \\
\text { C } & -e & -e \\
\text { D } & -e & 0 \\
\hline
\end{array}
\]
9. Question
1 point(s)
What is true for atoms of this nuclide?
10. Question
1 point(s)
What is the composition of one nucleus of this nuclide?
\begin{array}{|c|c|c|}
\hline & \text { neutrons } & \text { protons } \\
\hline \text { A } & 6 & 8 \\
\text { B } & 6 & 14 \\
\text { C } & 8 & 6 \\
\text { D } & 14 & 6 \\
\hline
\end{array}
\]
11. Question
1 point(s)
What is the proton number of a nucleus of this nuclide?
12. Question
1 point(s)
\begin{array}{|c|c|c|}
& \begin{array}{c}
\text { number of neutrons } \\
\text { remaining }
\end{array} & \begin{array}{c}
\text { number of protons } \\
\text { remaining }
\end{array} \\
\hline \text { A } & 142 & 93 \\
\text { B } & 142 & 95 \\
\text { C } & 144 & 93 \\
\text { D } & 144 & 95 \\
\hline
\end{array}
\]
13. Question
1 point(s)
14. Question
1 point(s)
15. Question
1 point(s)
What are the new nucleon and proton numbers?
\begin{array}{c|c|c|}
\hline & \text { nucleon number } & \text { proton number } \\
\hline \text { A } & 238 & 88 \\
\text { B } & 236 & 90 \\
\text { C } & 234 & 92 \\
\text { D } & 234 & 90 \\
\hline
\end{array}
\]
0 of 13 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 13 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) The nuclide symbol for radioactive polonium is \({ }_{84}^{210} \mathrm{Po}\). A nucleus of this type of polonium emits an \(\alpha\)-particle. The nuclide notation for radium-226 is \({ }_{88}^{226} \mathrm{Ra}\). A radioactive nucleus contains 128 nucleons. It emits a \(\beta\)-particle. A nuclide has the symbol \({ }_6^{14} \mathrm{C}\). A lithium nucleus contains 3 protons and 4 neutrons. A particular nuclide of chlorine can be represented by the symbol shown. A nuclide is represented by the symbol Which statement about the nuclei of all atoms is correct? The graph shows how the count rate on a detector due to a radioactive source changes with \(t\) The diagram represents a carbon atom. A nuclide is represented by the notation shown. A nucleus \(X\) has 17 protons and 18 neutrons. A nucleus of helium has the symbol \({ }_2^3 \mathrm{He}\).
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
What is the proton number (atomic number) of the nucleus after it has emitted the \(\alpha\)-particle?
2. Question
1 point(s)
How many electrons orbit the nucleus of a neutral atom of radium-226?
3. Question
1 point(s)
How many nucleons are now in the nucleus?
4. Question
1 point(s)
How many protons are there in one nucleus of this nuclide?
5. Question
1 point(s)
What is its nuclide notation?
6. Question
1 point(s)
\({ }_{17}^{37} \mathrm{Cl}\)
How many electrons are there in a neutral atom of this nuclide?
7. Question
1 point(s)
![]() .
.
How many neutrons are in one nucleus of the nuclide?
8. Question
1 point(s)
9. Question
1 point(s)
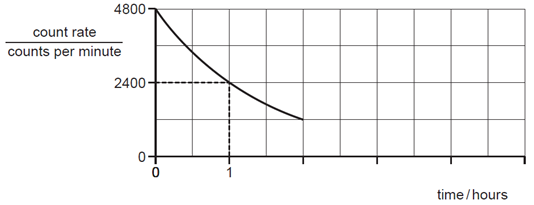
What is the count rate at 5.0 hours?
10. Question
1 point(s)
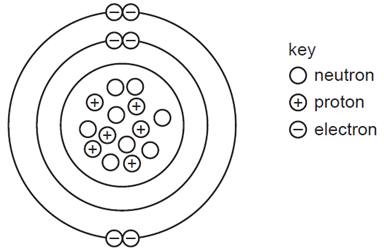
What is the nucleon number (mass number) for this atom?
11. Question
1 point(s)
![]()
How many nucleons are there in one atom of this nuclide?
12. Question
1 point(s)
Which notation is correct for this nucleus?
13. Question
1 point(s)
Which diagram represents an atom of \({ }_2^3 \mathrm{He}\) ?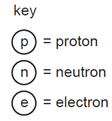
0 of 13 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 13 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Which diagram could represent the structure of a neutral atom? Which statement about a neutral atom of \({ }_{88}^{226} \mathrm{Ra}\) is correct? A radioactive nucleus emits \(\beta\)-particle An atom of the element lithium has a nucleon number of 7 and a proton number of 3 . A nuclide of substance \(X\) has the symbol \({ }_{12}^{26} X\). A nucleus of americium \({ }_{95}^{243} \mathrm{Am}\) emits an \(\alpha\)-particle to form a nucleus of neptunium (Np). A certain element has several isotopes. A very important experiment increased scientists’ understanding of the structure of matter. \[ Below are the symbols for five different nuclides. Below are four statements about isotopes of a certain element. Which diagram represents an experiment that provided evidence for the nuclear atom? The scattering of \(\alpha\)-particles by a thin metal foil supports the nuclear model of an atom. A reading is taken every 10 minutes of the number of emissions per second from a radioactive source. The table shows the readings.
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)

2. Question
1 point(s)
3. Question
1 point(s)
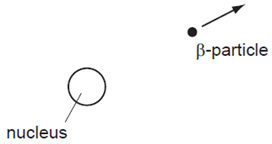
What happens to the proton number (atomic number) of the nucleus?
4. Question
1 point(s)
Which diagram represents a neutral atom of lithium?
5. Question
1 point(s)
How many electrons are there in a neutral atom of substance \(X\) ?
6. Question
1 point(s)
Which equation represents this decay?
7. Question
1 point(s)
Which statement about these isotopes is correct?
8. Question
1 point(s)
In the experiment, particles scattered as they passed through a thin metal foil.
Which particles were used, and to which conclusion did the experiment lead?
\begin{array}{|c|c|c|}
\hline & \text { particles } & \text { conclusion } \\
\hline \text { A } & \text { alpha particles } & \text { matter is made up of atoms } \\
\text { B } & \text { alpha particles } & \text { atoms have a very small nucleus } \\
\text { C } & \text { beta particles } & \text { matter is made up of atoms } \\
\text { D } & \text { beta particles } & \text { atoms have a very small nucleus } \\
\hline
\end{array}
\]
9. Question
1 point(s)
\[
\begin{array}{ccccc}
{ }_{17}^{35} \mathrm{X} & { }_{17}^{37} \mathrm{X} & { }_{18}^{38} \mathrm{X} & { }_{35}^{81} \mathrm{X} & { }_{37}^{81} \mathrm{X} \\
\text { nuclide 1 } & \text { nuclide 2 } & \text { nuclide 3 } & \text { nuclide 4 } & \text { nuclide 5 }
\end{array}
\]
Which two nuclides are isotopes of the same element?
10. Question
1 point(s)
Which statement about the isotopes must be correct?
11. Question
1 point(s)
12. Question
1 point(s)
Why are \(\alpha\)-particles used rather than neutrons?
13. Question
1 point(s)
\[
\begin{array}{|c|c|}
\hline \text { time/min } & \begin{array}{c}
\text { number of } \\
\text { emissions } \\
\text { per second }
\end{array} \\
\hline 0 & 800 \\
10 & 560 \\
20 & 400 \\
30 & 280 \\
40 & 200 \\
50 & 140 \\
60 & 100 \\
\hline
\end{array}
\]
What is the half-life of the source?
