Extra Questions
Quiz Summary
0 of 10 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 10
1. Question
Atoms and ions of metals P,Q and R take part in two reactions.
The equations for these reactions are shown.
P + 2Q+ → P2+ + 2Q
R + P2+ → R2+ +P
Which statements are correct?
- P is more reactive than R.
- P is less reactive than Q.
- R is more reactive than Q.
- R loses electrons most readily.
CorrectIncorrect -
Question 2 of 10
2. Question
25 cm³ of 0.1 mol/dm³ hydrochloric acid exactly neutralise 20 cm³ of aqueous sodium hydroxide. The equation for this reaction is:
NaOH + HCl → NaCl + H2O
What is the concentration of the sodium hydroxide solution?CorrectIncorrect -
Question 3 of 10
3. Question
A compound is analysed and found to contain 85.7% carbon and 14.3% hydrogen. What is its empirical formula?
CorrectIncorrect -
Question 4 of 10
4. Question
A compound, X, contains 40.0% carbon, 6.7% hydrogen and 53.3% oxygen by mass. The relative molecular mass, Mr, of X is 60.
What is the molecular formula of X?CorrectIncorrect -
Question 5 of 10
5. Question
A student does 10 J of work when lifting an object through a vertical distance of 2.0 m. What is the size of the force that the student exerts on the object?
CorrectIncorrect -
Question 6 of 10
6. Question
Carbon monoxide burns in oxygen to produce carbon dioxide.
2CO(g) + O2(g) → 2CO2(g)
Which mass of carbon dioxide is produced from 14g of carbon monoxide?CorrectIncorrect -
Question 7 of 10
7. Question
Rubidium is in Group I of the Periodic Table and bromine is in Group VII.
Rubidium reacts with bromine to form an ionic compound.
Which row shows the electron change taking place for rubidium and the correct formula of the rubidium ion?
\[
\begin{array}{|c|c|c|}
\hline & \text { electron change } & \text { formula of ion formed } \\
\hline \text { A } & \text { electron gained } & Rb ^{+} \\
\text {B } & \text { electron gained } & Rb ^{-} \\
\text {C } & \text { electron lost } & Rb ^{+} \\
\text {D } & \text { electron lost } & Rb ^{-} \\
\hline
\end{array}
\]CorrectIncorrect -
Question 8 of 10
8. Question
The arrangements of the electrons in two ions formed from elements X and Y are shown.

Which equation represents the reaction between elements X and Y?
CorrectIncorrect -
Question 9 of 10
9. Question
The diagram shows the speed-time graph for a moving object.
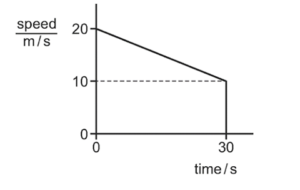
What is the distance travelled by the object in 30 s?
CorrectIncorrect -
Question 10 of 10
10. Question
The electronic structures of two atoms, \(P\) and \(Q\), are shown.
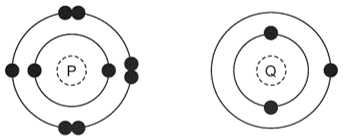 \(P\) and \(Q\) combine together to form a compound.
\(P\) and \(Q\) combine together to form a compound.
What is the type of bonding in the compound and what is the formula of the compound?
\[
\begin{array}{|c|c|c|}
\hline & \text { type of bonding } & \text { formula } \\
\hline \text { A } & \text { ionic } & PQ \\
\text { B } & \text { ionic } & PQ _2 \\
\text { C } & \text { covalent } & PQ _2 \\
\text { D } & \text { covalent } & PQ \\
\hline
\end{array}
\]CorrectIncorrect