6.2.1 Chemical Cell
Quiz Summary
0 of 4 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 4 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 4
1. Question
1 point(s)The diagram shows a simple cell.
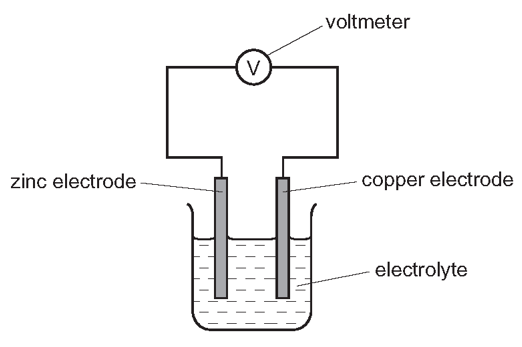
Which statement about the process occurring when the cell is in operation is correct?
CorrectIncorrect -
Question 2 of 4
2. Question
1 point(s)The diagram shows a simple cell.
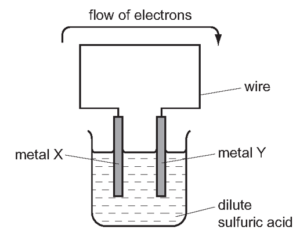
For which pair of metals would electrons flow from metal \(X\) to metal \(Y\) ?\[
\begin{array}{l}
\begin{array}{|c|c|c|}
\hline & \text { X } & Y \\
\hline \text { A } & \text { copper } & \text { iron } \\
\text { B } & \text { copper } & \text { zinc } \\
\text { C } & \text { iron } & \text { zinc } \\
\text { D } & \text { zinc } & \text { iron } \\
\hline
\end{array}
\end{array}
\]CorrectIncorrect -
Question 3 of 4
3. Question
1 point(s)The diagram shows a simple cell.
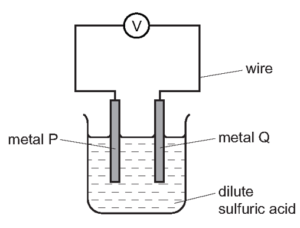
Which pair of metals produces the largest voltage?\[
\begin{array}{|c|c|c|}
\hline & \text { metal P } & \text { metal Q } \\
\hline \text { A } & \text { iron } & \text { copper } \\
\text { B } & \text { magnesium } & \text { copper } \\
\text { C } & \text { magnesium } & \text { zinc } \\
\text { D } & \text { zinc } & \text { copper } \\
\hline
\end{array}
\]CorrectIncorrect -
Question 4 of 4
4. Question
1 point(s)The reactivity series for a number of different metals is shown.
\[
\begin{array}{c c c}
\text { most reactive } \longrightarrow \text { least reactive }\\
\end{array}\]
\[\begin{array}{|c|c|c|c|c|c|}
\hline \text { magnesium } & \text { zinc } & \text { iron } & \text { copper } & \text { silver } & \text { platinum } \\
\hline
\end{array}
\]
The diagram shows different metal strips dipped into an electrolyte.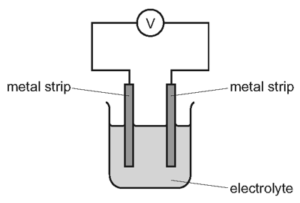
Which pair of metals produces the highest voltage?
CorrectIncorrect