0620/42_Summer_2023_Q1
(a) State which of the oxides, A to H, is responsible for acid rain.
Explanation: Sulfur dioxide reacts with water in the atmosphere to form sulfuric acid, contributing to acid rain.
(b) State which of the oxides, A to H, has a giant covalent structure.
Explanation: Silicon(IV) oxide has a network of strong covalent bonds forming a giant lattice structure.
(c) State which of the oxides, A to H, is a reducing agent in the blast furnace.
Explanation: Carbon monoxide reduces iron(III) oxide to iron in the blast furnace.
(d) State which of the oxides, A to H, is the main constituent of bauxite.
Explanation: Bauxite is primarily composed of aluminium oxide.
(e) State which of the oxides, A to H, is the main impurity in iron ore.
Explanation: Silicon(IV) oxide, also known as silica, is a common impurity in iron ore.
(f) State which of the oxides, A to H, can be reduced by heating with copper.
Explanation: Silver oxide can be reduced to silver metal by heating it with copper.
0620/42_Summer_2023_Q2
(a) State the name given to Group VII elements.
Explanation: Group VII elements are called halogens because they form salts when they react with metals.
(b) Explain why Group VII elements have similar chemical properties.
Explanation: The number of valence electrons determines an element's chemical properties, and all Group VII elements have seven valence electrons.
(c) Complete Table 2.1 to show the colour and state at r.t.p. of some Group VII elements.
\begin{align}
\begin{aligned}
&\text { Table } 2.1\\
&\begin{array}{|c|c|c|}
\hline \text { element } & \text { colour } & \text { state at r.t.p. } \\
\hline \text { fluorine } & \text { pale yellow } & \\
\hline \text { chlorine } & & \\
\hline \text { bromine } & & \text { liquid } \\
\hline
\end{array}
\end{aligned}
\end{align}
– Chlorine: pale yellow-green, gas
-Bromine: red-brown, liquid
Explanation: Fluorine and chlorine are gases at room temperature and have similar colors, while bromine is a liquid with a distinct red-brown color.
(d) Bromine has two naturally occurring isotopes, ⁷⁹Br and ⁸¹Br.
(i) State the term given to the numbers 79 and 81 in these isotopes of bromine.
Explanation: The nucleon number or mass number is the total number of protons and neutrons in an atom's nucleus.
(ii) Complete Table 2.2 to show the number of protons, neutrons and electrons in the atom and ion of bromine shown.
\begin{align}
\begin{aligned}
&\text { Table } 2.2\\
&
\end{aligned}
\end{align}
– ⁸¹Br⁻: 35 protons, 46 neutrons, 36 electrons
Explanation: The number of protons defines the element, while the number of neutrons varies between isotopes. For ions, the number of electrons changes to reflect the charge.
(iii) Table 2.3 shows the relative abundances of the two naturally occurring isotopes of bromine.
\begin{align}
\begin{aligned}
&\text { Table } 2.3\\
&\begin{array}{|l|c|c|}
\hline \text { isotope } & { }^{79} \mathrm{Br} & { }^{81} \mathrm{Br} \\
\hline \text { relative abundance } & 55 \% & 45 \% \\
\hline
\end{array}
\end{aligned}
\end{align}
Calculate the relative atomic mass of bromine to one decimal place.
Explanation: The relative atomic mass is calculated as (79 × 0.55) + (81 × 0.45) = 79.9.
(e) Chlorine displaces bromine from aqueous potassium bromide but does not displace fluorine from aqueous sodium fluoride.
(i) Write the symbol equation for the reaction between chlorine and aqueous potassium bromide.
Explanation: Chlorine, being more reactive, displaces bromine from potassium bromide, forming potassium chloride and bromine.
(ii) State why chlorine does not displace fluorine from aqueous sodium fluoride.
Explanation: Fluorine is the most reactive halogen, so chlorine cannot displace it from its compounds.
(f) Aqueous silver nitrate is a colourless solution containing Ag⁺(aq) ions.
(i) Describe what is seen when aqueous silver nitrate is added to aqueous sodium chloride.
Explanation: Silver chloride is formed, which is insoluble in water, resulting in a white precipitate.
(ii) Write the ionic equation for the reaction between aqueous silver nitrate and aqueous sodium chloride. Include state symbols.
Explanation: The reaction between silver ions and chloride ions forms solid silver chloride, which precipitates out of solution.
0620/42_Summer_2023_Q3
(a) State the name of the process used to manufacture sulfuric acid.
Explanation: The Contact process is widely used for the industrial production of sulfuric acid by oxidizing sulfur dioxide to sulfur trioxide, which is then converted to sulfuric acid.
(b) Part of the manufacture of sulfuric acid involves converting sulfur dioxide to sulfur trioxide.
(i) Describe two methods by which sulfur dioxide is obtained.
• Roasting sulfide ores (in air).
Explanation: Sulfur dioxide can be produced by burning sulfur in the presence of air or by roasting metal sulfide ores, which releases sulfur dioxide gas.
The conversion of sulfur dioxide to sulfur trioxide is a reversible reaction which can reach equilibrium.
(ii) State two features of an equilibrium.
• Concentrations of reactants and products are constant.
Explanation: At equilibrium, the rates of the forward and reverse reactions are equal, and the concentrations of the reactants and products remain constant over time.
(iii) State the typical conditions and name the catalyst used in the conversion of sulfur dioxide to sulfur trioxide.
• Pressure: 200 kPa.
• Catalyst: Vanadium(V) oxide.
Explanation: The optimal conditions for converting sulfur dioxide to sulfur trioxide include a temperature of about 450°C, a pressure of around 200 kPa, and the use of vanadium(V) oxide as a catalyst to increase the reaction rate.
(iv) Complete Table 3.1 to show the effect, if any, when the following changes are applied to the conversion of sulfur dioxide to sulfur trioxide.
\begin{align}
2 \mathrm{SO}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_3(\mathrm{~g})
\end{align}
The forward reaction is exothermic.
Only use the words increases, decreases or no change.
\begin{align}
\begin{aligned}
&\text { Table } 3.1\\
&\begin{array}{|c|c|c|}
\hline \text { change } & \begin{array}{c}
\text { effect on the rate of } \\
\text { the forward reaction }
\end{array} & \begin{array}{c}
\text { effect on the concentration } \\
\text { of } \mathrm{SO}_3(\mathrm{~g}) \text { at equilibrium }
\end{array} \\
\hline \text { temperature decreases } & \text { decreases } & \\
\hline \text { pressure increases } & & \\
\hline \text { no catalyst } & \text { decreases } & \\
\hline
\end{array}
\end{aligned}
\end{align}
• Pressure increases: Increases
• No catalyst: Decreases
Explanation: Decreasing the temperature slows down the reaction rate as the particles have less kinetic energy. Increasing the pressure increases the reaction rate due to more frequent collisions. Without a catalyst, the reaction rate decreases because catalysts provide an alternative pathway with a lower activation energy.
(v) Explain in terms of collision theory why reducing the temperature decreases the rate of the forward reaction.
• Frequency of collisions between particles decreases.
• Lower percentage of collisions have energy greater than or equal to activation energy.
Explanation: Reducing the temperature decreases the kinetic energy of the particles, resulting in fewer collisions and a lower proportion of collisions having enough energy to overcome the activation energy barrier.
(c) Sulfuric acid contains SO₄²⁻ ions.
The oxidation number of O atoms in SO₄²⁻ ions is –2. Determine the oxidation number of S atoms in SO₄²⁻ ions.
Show your working.
• S + (4 × –2) = –2 ∴ S = +6
Explanation: The total oxidation number of the SO₄²⁻ ion must equal the ion's charge, which is –2. Each oxygen atom has an oxidation number of –2, and there are four oxygen atoms. To balance this, sulfur must have an oxidation number of +6.
0620/42_Summer_2023_Q4
(a) State what is meant by the term base.
Explanation: A base can accept hydrogen ions (H⁺) from acids, thereby neutralizing them.
(b) State the term given to a base which dissolves to form an aqueous solution.
Explanation: Alkalis release hydroxide ions (OH⁻) when dissolved in water, making the solution alkaline.
(c) State the color of thymolphthalein in NaOH(aq).
Explanation: Thymolphthalein is an indicator that changes to blue in alkaline solutions like NaOH.
(d) Complete the word equation for the reaction of NaOH(aq) with ammonium chloride.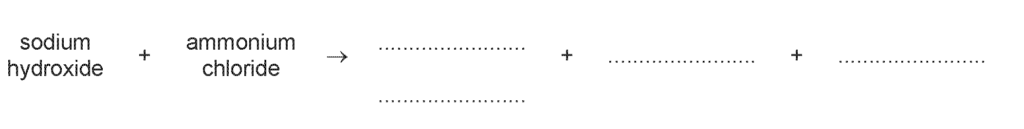
Explanation: This reaction is a neutralization reaction between a base (NaOH) and an ammonium salt (NH₄Cl), producing salt, water, and ammonia gas.
(e) Some metal oxides react with NaOH(aq).
(i) State the term given to metal oxides which react with bases such as NaOH(aq).
Explanation: Amphoteric oxides can react with both acids and bases.
(ii) Name a metal oxide which reacts with NaOH(aq).
Explanation: Both aluminium oxide and zinc oxide are amphoteric, meaning they can react with bases like NaOH.
(f) Ethanoic acid, CH₃COOH, is a weak acid.
(i) Complete the dot-and-cross diagram in Fig. 4.1 of a molecule of ethanoic acid.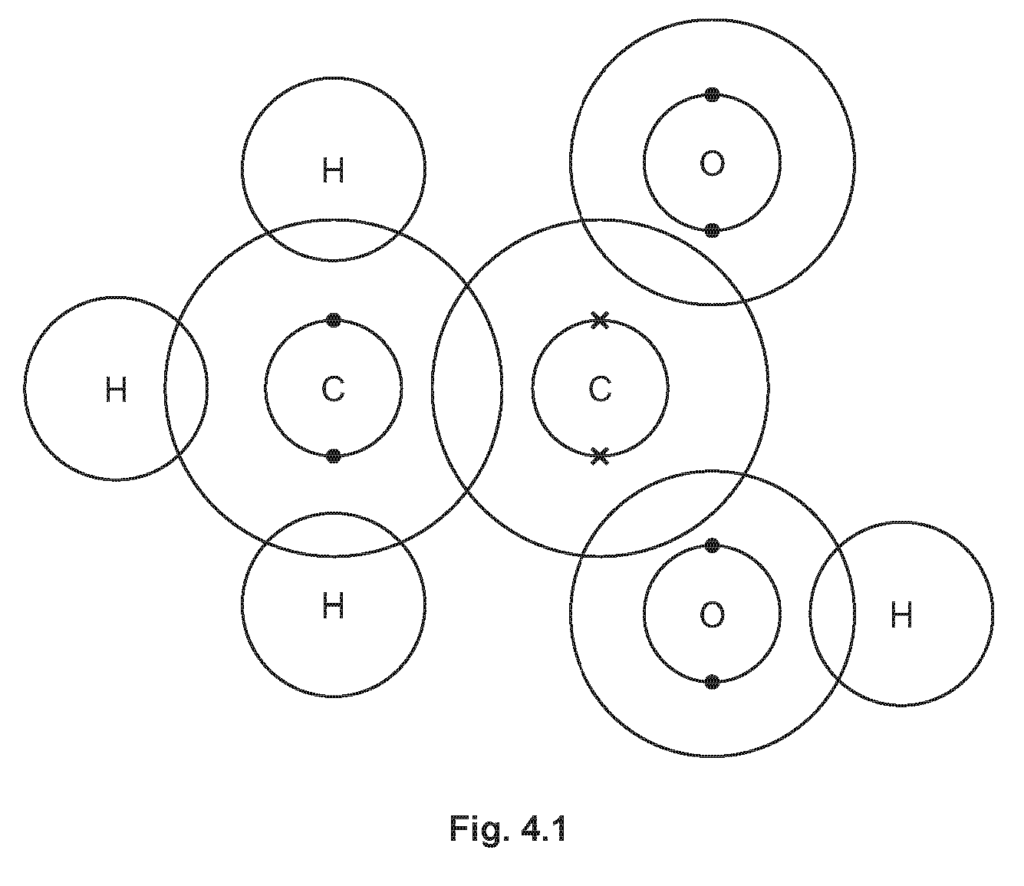
Explanation: The diagram should accurately represent the covalent bonding within the ethanoic acid molecule.
(ii) Suggest the pH of dilute ethanoic acid.
Explanation: Ethanoic acid is a weak acid, so it partially dissociates in solution, resulting in a pH less than 7 but greater than 3.
(iii) Complete the symbol equation to show the dissociation of ethanoic acid.
Explanation: The equation represents the dissociation of ethanoic acid in water, showing the equilibrium between the undissociated acid and its ions.[/bg_collapse]
(iv) Write the ionic equation for the reaction when an acid neutralizes a soluble base.
Explanation: This ionic equation represents the neutralization reaction between hydrogen ions from the acid and hydroxide ions from the base to form water.
(g) In a titration, 25.0 cm³ of 0.0800 mol/dm³ aqueous potassium hydroxide, KOH(aq), is neutralized by 20.0 cm³ of dilute sulfuric acid, H₂SO₄(aq).
\begin{align}
2 \mathrm{KOH}(\mathrm{aq})+\mathrm{H}_2 \mathrm{SO}_4(\mathrm{aq}) \rightarrow \mathrm{K}_2 \mathrm{SO}_4(\mathrm{aq})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})
\end{align}
Calculate the concentration of \(\mathrm{H}_2 \mathrm{SO}_4\), in \(\mathrm{g} / \mathrm{dm}^3\) using the following steps.
(i) Calculate the concentration of H₂SO₄, in g/dm³ using the following steps.
• Calculate the number of moles of KOH used.
Explanation: This is calculated using the formula moles = concentration × volume.
• Determine the number of moles of H₂SO₄ which react with the KOH.
Explanation: Since the balanced equation shows that one mole of H₂SO₄ reacts with two moles of KOH, we divide the moles of KOH by 2.
• Calculate the concentration of H₂SO₄ in mol/dm³.
Explanation: The concentration is calculated using the formula concentration = moles / volume.
• Calculate the concentration of H₂SO₄ in g/dm³.
Explanation: This is found by multiplying the molar concentration by the molar mass of H₂SO₄ (98 g/mol).
0620/42_Summer_2023_Q5
When a molecule of propane, C₃H₈, reacts with chlorine in the presence of ultraviolet light, one atom of hydrogen is replaced by one atom of chlorine.
(i) State the term given to reactions in which one atom in an alkane is replaced by another atom.
Explanation: Substitution reactions involve the replacement of an atom or group of atoms in a molecule with a different atom or group of atoms.
(ii) State the purpose of ultraviolet light in this reaction.
Explanation: Ultraviolet light provides the necessary energy to break bonds, initiating the chemical reaction.
(iii) State the term given to any reaction which requires ultraviolet light.
Explanation: Photochemical reactions are driven by light energy, typically involving ultraviolet light to initiate the process.
(iv) Write the symbol equation for the reaction between propane and chlorine.
Explanation: In this reaction, chlorine replaces a hydrogen atom in propane, forming chloropropane and hydrogen chloride.
A molecule of propene, C₃H₆, is unsaturated and will react with chlorine at room temperature.
(i) State why propene is an unsaturated molecule.
Explanation: Unsaturated molecules contain double or triple bonds between carbon atoms, allowing for additional chemical reactions.
(ii) Give the structural formula of the product of this reaction.
Explanation: When propene reacts with chlorine, the double bond breaks, and each carbon atom that was part of the double bond gains a chlorine atom.
Propene undergoes addition reactions with steam. There are two possible products, A and B.
Draw the displayed formula and name each product.
displayed formula of product A
name of product A
displayed formula of product B
name of product B
– Product A: Propan-1-ol (CH₃CH₂CH₂OH)
– Product B: Propan-2-ol (CH₃CH(OH)CH₃)
Explanation: The addition of steam to propene can result in two different alcohols depending on the position where the hydroxyl group (-OH) attaches to the carbon chain.
0620/42_Summer_2023_Q6
(a) Name the ester formed when butanoic acid, CH₃CH₂CH₂COOH, reacts with ethanol, CH₃CH₂OH.
Explanation: This ester is named by combining the alkyl group from the alcohol (ethyl) and the acid part (butanoate).
(b) Identify the other product formed in this reaction.
Explanation: In esterification, water is formed as a by-product when an alcohol reacts with a carboxylic acid.
(c) Deduce the empirical formula of the ester formed.
Explanation: The molecular formula of ethyl butanoate is C₆H₁₂O₂. Simplifying this gives the empirical formula C₃H₆O.
(d) PET is a polyester. Part of the structure of PET is shown in Fig. 6.1.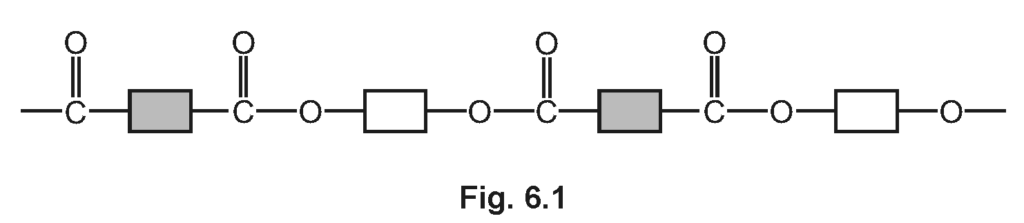
(i) Circle one repeat unit of this polymer.
Explanation: A repeat unit in a polymer is the specific arrangement of atoms that repeats throughout the polymer chain.
(ii) Draw the structures of the monomers which make up PET. Draw the functional groups using displayed formulae.
• Ethylene glycol (HO-CH₂-CH₂-OH)
• Terephthalic acid (HOOC-C₆H₄-COOH)
Explanation: PET is formed from the condensation polymerisation of ethylene glycol and terephthalic acid.
(iii) State the type of polymerisation used in making PET.
Explanation: Condensation polymerisation involves the joining of monomers with the loss of a small molecule, such as water.
0620/42_Winter_2023_Q1
(a) State which of the atoms or ions, A to G, could be a noble gas atom.
Explanation: A noble gas has a full outer shell of electrons. The electronic configuration 2,8 represents neon, which is a noble gas.
(b) State which of the atoms or ions, A to G, could be an atom of an element in Group VI.
Explanation: Group VI elements have six electrons in their outer shell. The electronic configuration 2,8,6 corresponds to such an element, which is sulfur in this case.
(c) State which of the atoms or ions, A to G, could be an atom with an atomic number of 14.
Explanation: The atomic number is the total number of protons (and electrons in a neutral atom). The configuration 2,8,4 gives a total of 14 electrons, corresponding to silicon.
(d) State which of the atoms or ions, A to G, could be atoms from the same group.
Explanation: Atoms in the same group have the same number of electrons in their outer shell. Both A (2,5) and E (2,8,5) have 5 electrons in their outer shell.
(e) State which of the atoms or ions, A to G, could be a halogen atom.
Explanation: Halogens are in Group VII and have 7 electrons in their outer shell. The electronic configuration 2,8,18,7 corresponds to chlorine.
(f) State which of the atoms or ions, A to G, could be an atom of an element which is a good conductor of electricity.
Explanation: Metals are good conductors of electricity. The electronic configuration 2,8,2 corresponds to magnesium, which is a metal.
(g) State which of the atoms or ions, A to G, could be a stable ion of a Group V element.
Explanation: Group V elements form stable ions by gaining three electrons to have a full outer shell. The configuration 2,8 represents the nitrogen ion (N³⁻).[%bg_collapse]
(h) State which of the atoms or ions, A to G, could be an atom that forms an ion with a 2– charge.
Explanation: Atoms that form 2– ions gain two electrons to achieve a full outer shell. The configuration 2,8,6 represents sulfur, which forms S²⁻.
